Abstract
INTRODUCTION
Usage of metformin is associated with improved survival in lung, breast and prostate cancer, and metformin has been shown to inhibit cancer cell growth and proliferation in in vitro studies. Given the lack of clinical data on metformin use in patients with bladder cancer, we aimed to evaluate the role of metformin in their oncological outcomes.
METHODS
Medication use data from a prospectively maintained database of 122 patients with non-muscle-invasive bladder cancer treated with intravesical Bacille Calmette-Guerin (BCG), who were recruited under a randomised, double-blinded, controlled clinical trial, was collected and analysed. Kaplan-Meier curves were used to assess overall survival (OS) and disease-specific survival (DSS).
RESULTS
At a median follow-up duration of 102 (range 3–357) months, 53 (43.4%) patients experienced disease recurrence and 21 (17.2%) experienced disease progression. There was no significant difference in mortality between patients with and without diabetes mellitus. There was significant difference in OS between patients without diabetes mellitus, patients with diabetes mellitus on metformin and patients with diabetes mellitus but not on metformin (p = 0.033); patients with diabetes mellitus on metformin had the best prognosis. Metformin use was associated with significantly lower DSS (p = 0.042). Other oral hypoglycaemic agents, insulin or statins were not associated with disease recurrence or progression.
CONCLUSION
Metformin use was associated with improved oncological outcomes in patients with non-muscle-invasive bladder cancer treated with intravesical BCG. Prospective studies with larger patient populations are needed to validate the role of metformin as potential therapy for bladder cancer.
INTRODUCTION
A recent report by the International Diabetes Federation showed that Singapore has the second highest proportion of patients with diabetes mellitus among developed nations.(1) About 10.53% of the population in Singapore in the age range of 20–79 years was estimated to have the disease. Metformin, a biguanide, is one of the only two antidiabetic drugs on the World Health Organization list of essential medicines and one of the most commonly used drugs to treat non-insulin-dependent diabetes mellitus in Singapore. The drug’s glucose-lowering, cardioprotective effect and its relatively high benefit-risk ratio have made it a popular candidate of study among researchers looking to expand its uses.
Many studies have suggested an association between metformin use and improved survival in patients with lung, breast and prostate cancer.(2) Mechanistically, metformin is hypothesised to exert its antitumour effect by various mechanisms, such as the liver kinase B1-dependent and AMP-activated protein kinase-dependent suppression of mammalian target of rapamycin (mTOR) pathways.(3,4) Reduced cell proliferation and expression of downstream mediators of the mTOR pathway with metformin has been demonstrated in bladder carcinoma cell line studies.(5) However, there is a paucity of clinical data on the utility of metformin in patients with bladder cancer. Therefore, this study aimed to evaluate the role of metformin in the oncological outcomes of these patients.
METHODS
Information was collected from a prospectively maintained database of 122 patients with non-muscle-invasive bladder cancer, who were recruited under a Good Clinical Practice standard, randomised, double-blinded, controlled clinical trial. Informed consent was obtained from all participants in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The original study was approved by the institution’s Domain-Specific Review Board (reference no. DSRB 2006/00254).
All patients had undergone transurethral resection of bladder tumour between 1995 and 2013, and had received postoperative intravesical Bacillus Calmette-Guerin (BCG) instillations. All patients received the full standard induction of six one-weekly BCG instillations and at least three booster doses, except for two patients who were unable to tolerate the full regime and underwent three and four instillations, respectively. One of these two patients had diabetes mellitus and was on metformin, while the other did not have diabetes mellitus.
The inclusion criteria were patients with completely resected, histologically proven intermediate or high-risk non-muscle-invasive urothelial carcinoma of the urinary bladder and Karnofsky score > 50. The exclusion criteria were immunodeficiency, transitional cell carcinoma of stage T2 or higher, transitional cell carcinoma of the upper urinary tract or active tuberculosis.
All patients were generally followed up in accordance with the European Association of Urology guidelines for non-muscle-invasive bladder cancer. Follow-up comprised history-taking, physical examination, urinary cytology and cystoscopies.
Metformin use, clinical data and survival data were collected from the hospital’s computerised health records system. The primary exposure definition was use of metformin, defined as receiving at least one prescription between cohort entry and the last date of follow-up. For the purposes of secondary analysis, we also collected the duration and doses of metformin use.
Characteristics of patients with and without diabetes mellitus were compared using chi-square test for categorical variables and t-test for continuous variables. Current knowledge from literature reviews and causal diagrams were used to further analyse for potential confounders, such as cardiovascular risk factors, other oral hypoglycaemic agents and statins. Kaplan-Meier survival curves were employed to compare disease-specific survival (DSS) and overall survival (OS) for patients with and without diabetes mellitus. Data collected was analysed using PASW Statistics version 18.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
The study population comprised 122 patients with non-muscle-invasive bladder cancer. The mean age of the patients was 64.7 ± 11.1 years and the majority (82.8%) were men. The percentage of Chinese (81.1%) patients with bladder cancer was proportionately higher, similar to the ethnic composition of the Singapore population, while Malay (9.8%) and Indian (7.4%) patients accounted for a minority. 22 (18.0%) patients had diabetes mellitus, nine of whom were on metformin. The demographics of our study population are shown in
Table I
Characteristics of patients with non-muscle-invasive bladder cancer.
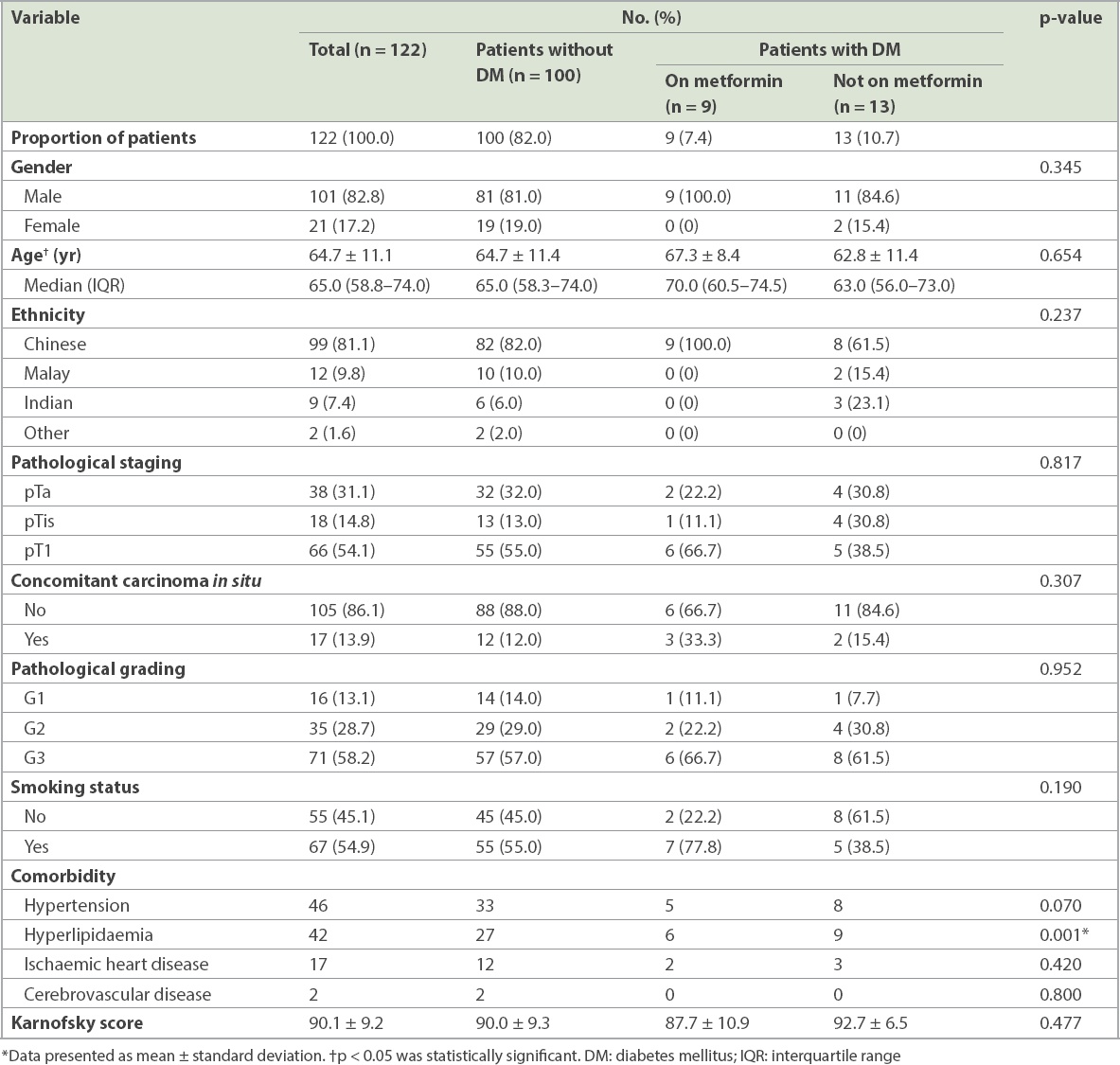
There was no significant difference between the three groups of patients in terms of demographics, smoking status, and pathological grading and staging of disease. A higher proportion of patients with diabetes mellitus (59.1%) had hypertension when compared to those without diabetes mellitus (33.0%). This was likely attributable to the association of diabetes mellitus with a patient’s cardiovascular health status. Similarly, patients with diabetes mellitus (68.2%) were significantly more likely than patients without diabetes mellitus (27.0%) to have a history of hyperlipidaemia (p = 0.001). There was no significant difference between the three groups in terms of mean Karnofsky score or other comorbidities.
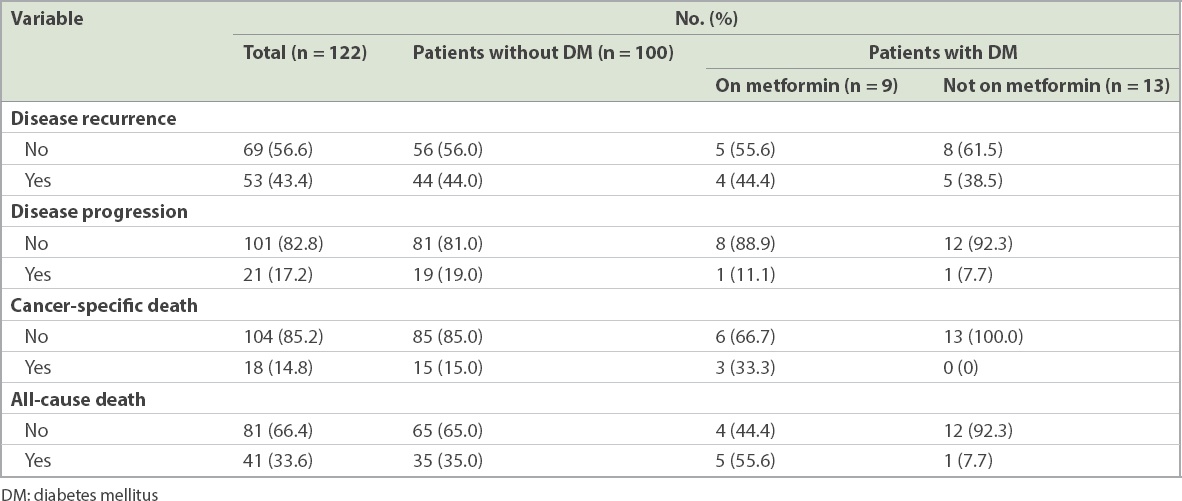
Patient outcomes included OS, DSS, recurrence-free survival and progression survival (
Table II
Outcomes of patients with non-muscle-invasive bladder cancer.
Figs.
Fig. 1
Kaplan-Meier curve shows overall survival (OS) of patients with non-muscle-invasive bladder cancer with and without DM. DM: diabetes mellitus
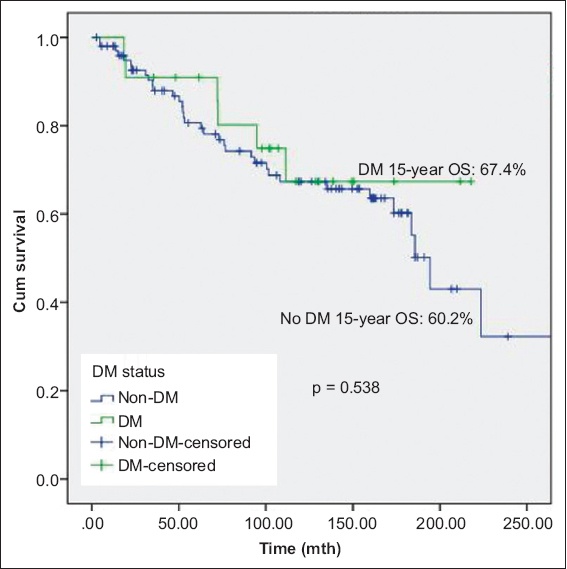
Fig. 2
Kaplan-Meier curve shows disease-specific survival (DSS) of patients with non-muscle-invasive bladder cancer with and without DM. DM: diabetes mellitus
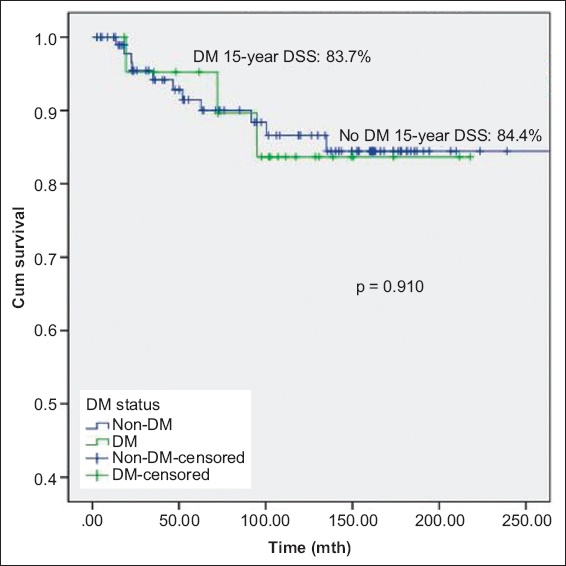
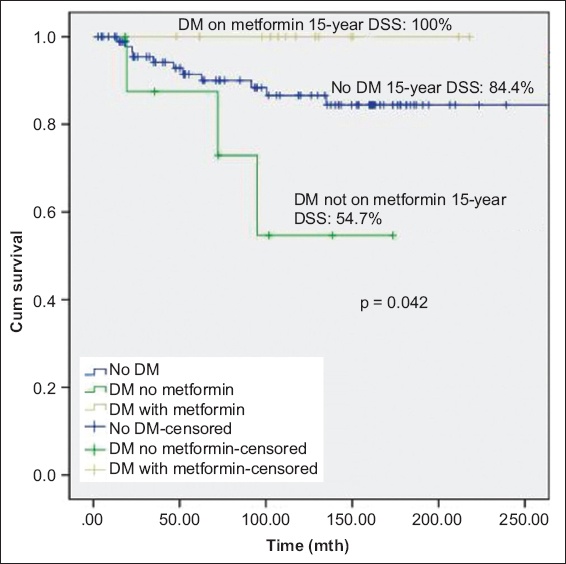
A secondary analysis, performed to explore the effects of duration of exposure, found significant differences in DSS after exposure to metformin for 15 years. There was significant difference in DSS (p = 0.042) between patients without diabetes mellitus, patients with diabetes mellitus on metformin and patients with diabetes mellitus not on metformin; patients with diabetes mellitus treated with metformin had the best prognosis (DSS 100.0% at 15 years;
Fig. 3
Kaplan-Meier curve shows disease-specific survival (DSS) of patients with non-muscle-invasive bladder cancer without DM, with DM on metformin, and with DM but not on metformin. DM: diabetes mellitus
DISCUSSION
One of the proposed mechanisms for the antitumour properties of metformin involves the inhibition of the phosphatidylinositol 3-kinase (PI3k)/protein kinase B (Akt)-mTOR pathway. Recent data suggested that the PI3k-Akt-mTOR signalling pathway plays a central role in controlling bladder cancer cell growth.(6) This is further corroborated by studies showing that 55% of human bladder tumours had increased expression of phosphorylated AKT.(7) In vitro, mTOR inhibition has been shown to inhibit the growth of bladder cancer cells in a dose-dependent manner.(8) In addition, the combination of metformin and other mTOR inhibitors have shown a synergistic effect on the inhibition of cell proliferation in other cell lines.(9,10)
The antiproliferative actions of metformin are hypothesised to manifest via the inhibition of the activation of AKT1, mTOR and insulin-like growth factor 1 receptor among other downstream signalling mediators. Further evidence for a potential role of metformin in the PI3k-Akt-mTOR pathway was also presented by Wang and Wu,(11) who found that treating bladder cancer cell lines T24 and BIU-87 with metformin or cisplatin resulted in reduced cell proliferation and downregulation of phosphorylated-mTOR on Western blot arrays. Zhang et al reported similar findings of inhibited proliferation and colony formation in cell lines 5637 and T24 with metformin, and demonstrated inhibition of tumour growth in a xenograft model with daily dosing of metformin.(5)
The results of our study are concordant with the in vitro and clinical data on the association between metformin use and bladder cancer recurrence risk. Multiple studies have shown that patients with diabetes mellitus who are not on metformin have an increased incidence of bladder cancer and, particularly, cancers with poorer oncological outcomes.(12,13) Our findings follow the general trend that has been demonstrated in these previous studies. Metformin is a cheap and popular drug commonly prescribed by Singapore physicians for patients with diabetes mellitus. Given the high prevalence of exposure, it was ideal to study the effect of metformin within our population. Additionally, we considered the effect of duration of exposure to metformin, which has not commonly been studied in previous studies.
However, our study was not without limitations. As it was a retrospective analysis of prospectively collected data, potential confounders such as smoking status and compliance to medication could not be recorded or analysed. However, we extensively evaluated the effects of potential confounders such as cardiovascular risk factors (e.g. hypertension and hyperlipidaemia) and the use of statins, which revealed no associations with the DSS and OS of our patient population. Selection bias was another inherent issue. Our patients with diabetes mellitus on metformin were restricted to those who received treatment at a tertiary cancer hospital, and may not have been reflective of the general population with diabetes mellitus. The small sample size of patients with diabetes mellitus on metformin was also another limitation.
However, it should be noted that most studies reflecting such associations are retrospective in nature and are, hence, prone to methodological limitations that should be taken into account when interpreting our results. Notably, a study based on a cohort of 87,600 patients in the United States(14) did not find a reduced incidence of bladder carcinoma with metformin use. Data from randomised controlled trials by Colmers et al(15) and Lewis et al(16) also did not reflect any additional protective benefit of metformin over rosiglitazone. However, the number of bladder cancer patients in both these earlier studies (n < 10) was modest at best, and caution should be exercised when arriving at a conclusion from the data. Furthermore, the performance of active comparator studies could indicate that although both metformin and rosiglitazone reduce the risk of bladder malignancy, one may not be better than the other.
A meta-analysis by Hu et al(17) found that metformin intake could improve the prognosis of patients with bladder cancer. This was concordant with our data, but the data in the meta-analysis was recruited from retrospective cohort studies and potential confounders such as gender, age and smoking status were not considered.
In conclusion, our study supported the association of improved DSS and OS in patients with diabetes mellitus on metformin with non-muscle-invasive bladder carcinoma treated with intravesical BCG. As existing studies in the literature have indicated conflicting results, prospective studies specifically designed to address the matter should aim to validate the role of metformin as potential therapy for non-muscle-invasive bladder carcinoma.
About the First Author

Dr Wang Ziting obtained her medical degree from the National University of Singapore in 2012 and gained fellowship with the Royal College of Surgeons of Edinburgh in 2014. She completed her Master of Medicine (Surgery) in 2017 and received specialist accreditation for Urology in 2020. Dr Wang’s clinical and research interests are in cancer survivorship and device innovation. She has won several grants for her research. For her work, she was awarded the National Outstanding Clinician Scientist Resident Award in 2019.


