Abstract
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) is increasingly performed in patients with severe aortic stenosis. A novel dual-filter system to reduce cerebral embolism during TAVI recently became available. We aimed to assess the feasibility, safety, and clinical and neurocognitive outcomes of TAVI with cerebral protection in Asian patients.
METHODS
40 consecutive patients undergoing TAVI with cerebral protection were enrolled. All procedures were performed via femoral access using the self-expanding Evolut R/PRO or Portico, or the balloon-expandable SAPIEN 3 bioprostheses. Baseline characteristics, procedural and clinical outcomes were recorded. Cognition was assessed at baseline and 30 days using the abbreviated mental test (AMT).
RESULTS
The mean age of the patients (75% male) was 76.4 ± 8.4 years. TAVI was uncomplicated in all patients. The filter device was successfully deployed in 38 (95.0%) patients without safety issues. There was no stroke or death at 30 days, and the survival rate at nine months was 95.0%. There was no overall cognitive change (baseline vs. 30-day AMT: 9.2 ± 1.1 vs. 9.0 ± 1.5, p = 0.12), and only 1 (2.5%) patient developed impaired cognition at 30 days. Patients with a decreased AMT score at 30 days were significantly older than those without (82.1 ± 4.5 vs. 74.4 ± 7.7 years, p = 0.019). All patients with decreased AMT scores were aged ≥ 76 years.
CONCLUSION
In this early Asian experience of TAVI under cerebral protection, the filter device was successfully deployed in 95% of patients, with 100% procedural success. There were no filter-related complications and no stroke or mortality at 30 days. Overall cognition was preserved, although increased age was associated with a decline in AMT score.
INTRODUCTION
Percutaneous transcatheter aortic valve implantation (TAVI), also known as transcatheter aortic valve replacement, has become an established treatment for patients with severe aortic valve stenosis (AS) who are inoperable or at high surgical risk,(1-3) and an attractive alternative in intermediate-risk patients.(4,5) More recently, randomised trials in low surgical-risk patients showed that TAVI was superior to surgical aortic valve replacement with regard to stroke or death at 1–2 years.(6,7) Although the incidence of stroke has declined from 2.5%–5% in the early years(8) to 0.6%–3.4% more recently,(6,7,9) it remains a concern. Neurocognitive decline can occur after surgical aortic valve replacement,(10) and this has also been documented in patients undergoing TAVI.(11) With TAVI now being performed in younger patients who are expected to be more active and have longer life expectancies, further reducing the stroke risk during TAVI and preserving higher cognitive function after TAVI have become increasingly important. Recently, a novel dual-filter device – the SENTINEL cerebral protection system (CPS) (Boston Scientific, Marlborough, MA, USA) – showed effectiveness in capturing cerebral emboli during TAVI(12-14) and reducing periprocedural stroke.(15,16) In this study, we aimed to assess the feasibility, safety, and the clinical and neurocognitive outcomes of TAVI under cerebral protection with the SENTINEL CPS.
METHODS
A total of 40 consecutive patients undergoing TAVI owing to symptomatic severe AS in a single centre were included. This was an all-comer study, and patients were enrolled regardless of their surgical risk profile. All patients were reviewed by a cardiologist and a cardiac surgeon and underwent pre-procedure computed tomography (CT) for detailed assessment of the great vessels, particularly tortuosity and size (to determine suitability for the SENTINEL CPS), aortic root (to determine valve type and size) and the peripheral arterial anatomy (to determine suitability for TAVI via femoral access). Carotid ultrasonography scans were not performed, as CT provided detailed anatomical assessment to the level of the carotid bifurcation.
All TAVI procedures were performed using the self-expanding Evolut R or Evolut PRO (Medtronic, Dublin, Ireland) prosthesis (
Fig. 1
Photograph shows the Evolut PRO self-expanding transcatheter heart valve (the Evolut R valve is similar but without the bottom external skirt denoted by the open bracket).
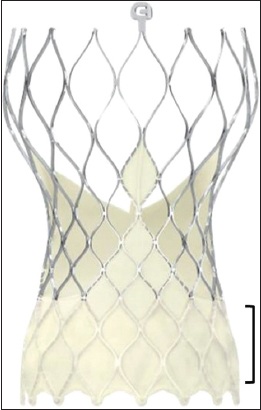
Fig. 2
Photograph shows the Edwards SAPIEN 3 balloon-expandable valve.
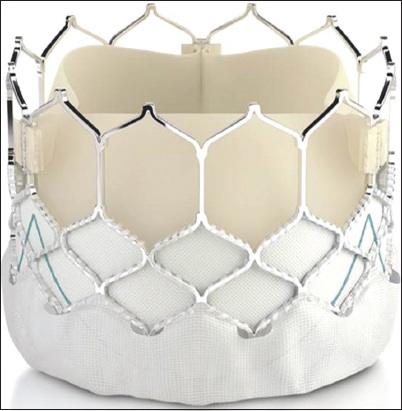
Fig. 3
Photograph shows the Portico valve, a self-expanding valve.

The filter device was the SENTINEL CPS (Boston Scientific, Marlborough, MA, USA) (
Fig. 4
Photographs show the SENTINEL cerebral protection system’s (a) filters, consisting of the proximal filter (arrow) and distal filter (arrowhead); and (b) delivery system.
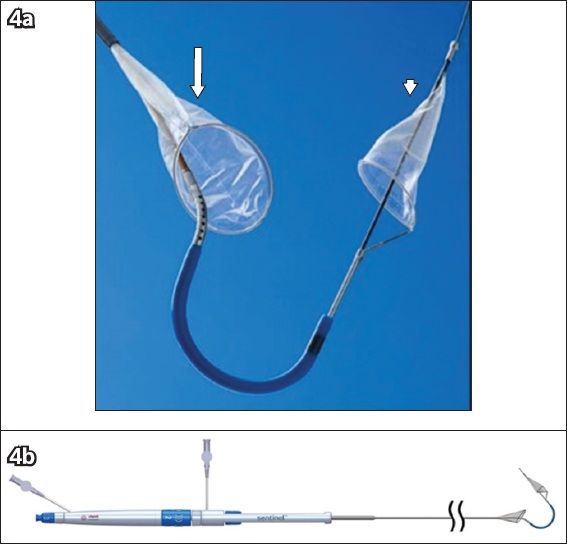
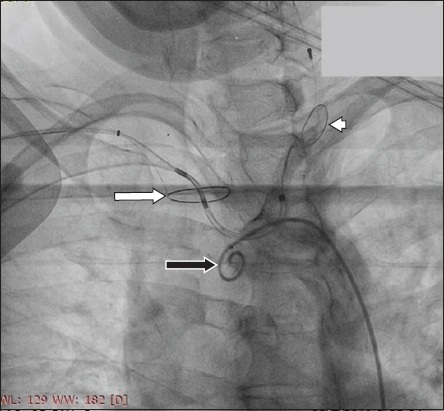
Fig. 5
Fluoroscopic image shows the SENTINEL proximal filter in the innominate artery (arrow) and the distal filter in the left carotid artery (arrowhead). A pigtail catheter (black arrow) can be seen in the arch of the aorta.
Patient characteristics, procedural parameters, and in-hospital, 30-day and nine-month outcomes were recorded. Procedural success (defined as successful transcatheter valve implantation in the correct position without major complications and without moderate or more than moderate paravalvular leak) and all adverse clinical outcomes and events were defined according to the Valve Academic Research Consortium-2 (VARC-2) consensus document.(17) Cognitive assessment, using the abbreviated mental state (AMT) test, was performed by a healthcare staff who was not involved in this study on all patients before and at 30-day follow-up after TAVI. This questionnaire assesses patients on ten questions, with a score of seven and below indicating neurocognitive impairment.(18,19) The primary endpoint was either stroke or death at 30 days after TAVI, and the secondary end point was cognitive impairment, as defined by an AMT score of seven or less after TAVI.
Continuous variables were presented as mean ± standard deviation and were compared between groups using a paired t-test. Categorical variables were given as frequencies and percentages and were compared by chi-square test or Fisher’s exact test. A p-value < 0.05 was considered statistically significant. Statistical analyses were performed with IBM SPSS Statistics version 23.0 (IBM Corp, Armonk, NY, USA). All patients provided informed consent for the study.
RESULTS
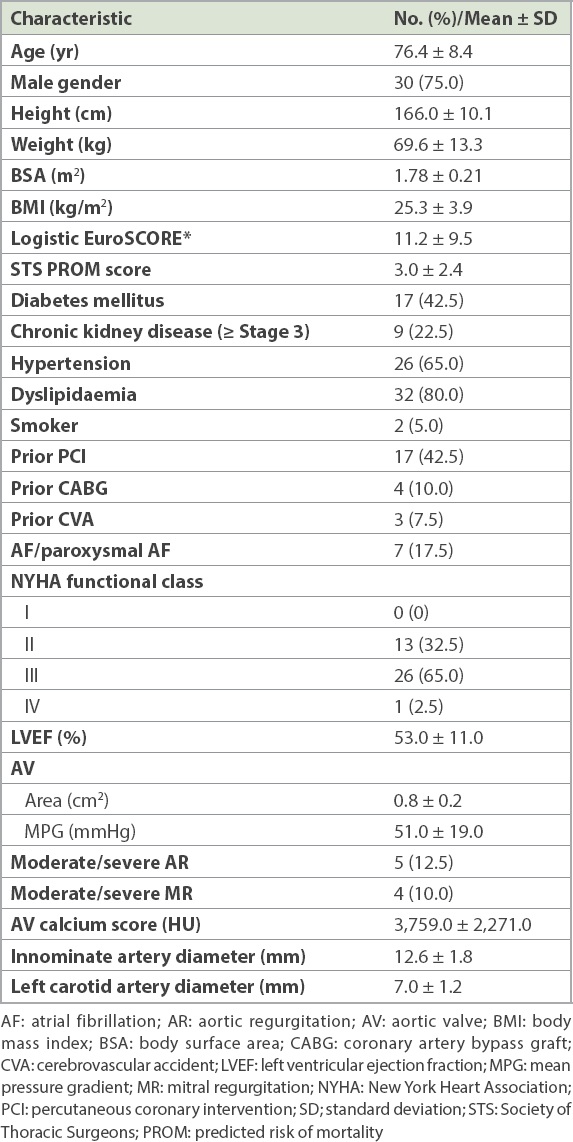
The mean age of our patients was 76.4 ± 8.4 years, and 30 (75.0%) of them were male. The mean left ventricular ejection fraction was 53% ± 11%, and nearly a quarter of patients had chronic kidney disease (≥ Stage 3). Of note, 3 (7.5%) patients had had a previous stroke with good functional recovery. There were two patients with bovine aortic arches and no other arch variants. Various degrees of calcified plaques in the ascending aorta and aortic arch were present in all patients; none, however, had a porcelain aorta, as defined by the VARC-2 consensus document. The baseline characteristics of the patients are summarised in
Table I
Baseline patient characteristics (n = 40).
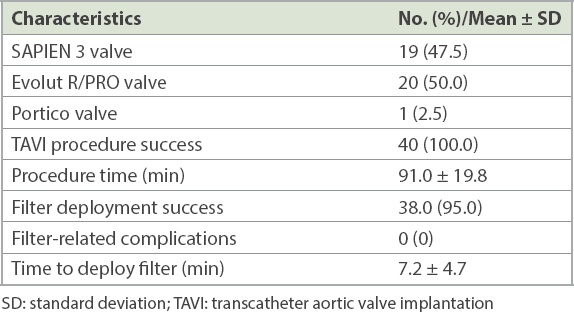
All TAVI procedures were successful; the Evolut R or PRO bioprosthesis was used in 20 (50.0%) patients, the SAPIEN 3 bioprosthesis was used in 19 (47.5%) patients and the Portico bioprosthesis was used in the remaining patient. The SENTINEL CPS was successfully deployed in 38 (95.0%) patients (including both patients with bovine aortic arches), with macroscopic debris (
Fig. 6
Photograph shows typical debris captured during the transcatheter aortic valve implantation procedure.
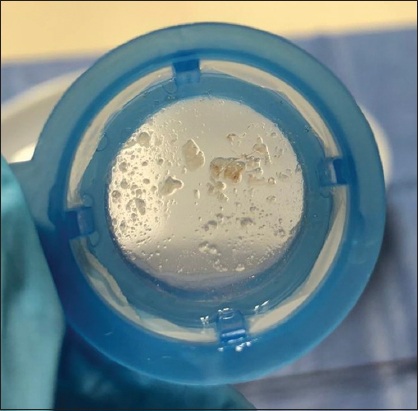
Table II
Procedural characteristics (n = 40)
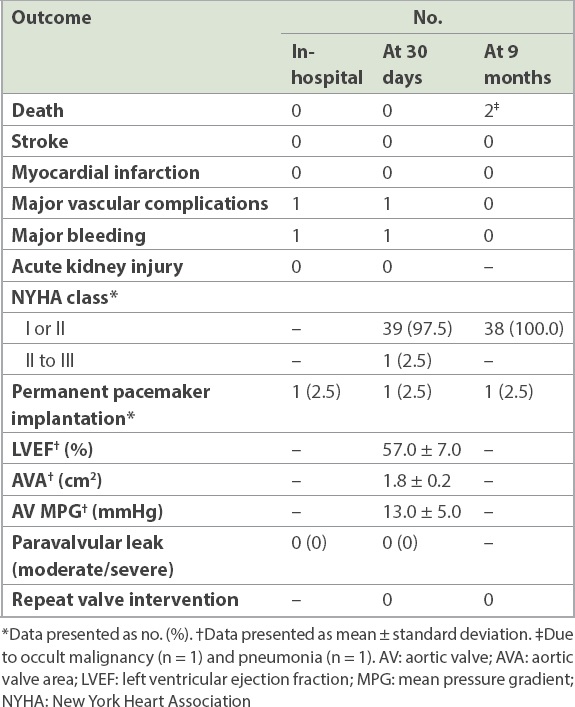
At 30 days, no stroke or death was observed. All patients reported significant functional status improvement, with almost all patients in New York Heart Association (NYHA) Class I or II. All patients had mild or less than mild paravalvular leak, and 1 (2.5%) patient required a permanent pacemaker implantation. The survival rate at nine months was 95.0% (38 out of 40 patients). One patient suffered from an occult gastric malignancy that was not detected at the time of TAVI and died four months after the procedure. Another patient developed severe sepsis due to pneumonia and died at three months. The clinical and haemodynamic outcomes are shown in
Table III
Clinical outcomes (n = 40).
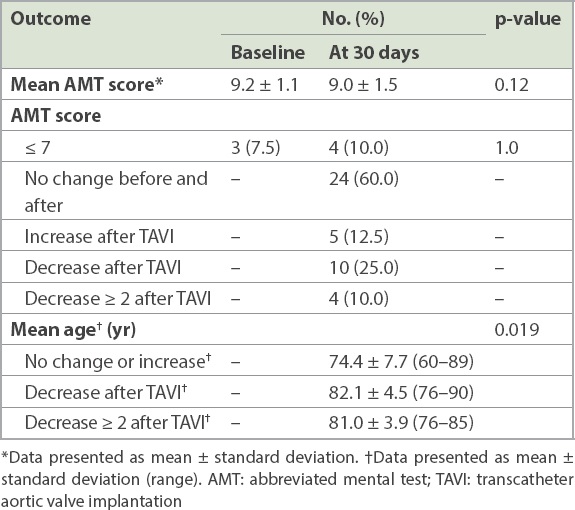
The cognitive outcomes are shown in
Table IV
Cognitive outcomes (n = 40).
DISCUSSION
This study describes the first experience of TAVI with cerebral protection in Southeast Asia, using a novel dual-filter device. The main findings were as follows: the TAVI procedure was successful in all patients, and the SENTINEL CPS was deployed in the intended locations in 95.0% of cases. The time required to deploy the SENTINEL device was under ten minutes, and there were no device-related complications. There was no in-hospital or 30-day stroke or mortality, and survival at nine months was 95.0%. Overall, cognition was preserved, with a similar mean AMT score at baseline and 30 days (9.2 ± 1.1 vs. 9.0 ± 1.5, p = 0.12). Four patients had cognitive impairment at 30 days, as compared with three at baseline (p = 1.0). Patients who showed a decrease in AMT score were significantly older, and no patients younger than 76 years experienced a decline in AMT score.
Since TAVI was first performed in Asia and Singapore in 2009,(20) indications have evolved and the case volume has increased throughout Asia.(21) Improvements in valve design and delivery systems have improved clinical outcomes, especially regarding residual paravalvular leak and permanent pacemaker requirements. With TAVI now being performed for lower-surgical-risk and younger patients, stroke risk and neurocognitive preservation have become increasingly important.
Studies using diffusion-weighted MR imaging showed that TAVI resulted in new brain lesions in 68%–84% of cases, and these occurred in both hemispheres and in both anterior and posterior circulations, consistent with cerebral embolisation.(8,22,23) Although the recent clinical stroke rate is < 3%, more concerning is the fact that silent brain infarcts have been associated with cognitive decline and dementia.(24)
Various cerebral protection devices have been developed to reduce the embolic burden to the brain during TAVI, with SENTINEL CPS being currently the only device that is FDA approved and has a CE mark. It is designed to capture embolic material – documented to consist of thrombus, calcification, valve tissue, artery wall and foreign material(12,25) – and has been shown to reduce the number and volume of cerebral emboli.(12-14)
While the SENTINEL CPS has been shown to be a safe device, with successful positioning of the filters in 92%–97% of cases, it increases the procedure time by 7–13 minutes and fluoroscopic time by 1.5–3 minutes.(12-14,16,26) Failures to deploy the SENTINEL CPS were due to significant innominate or right subclavian tortuosity or other complex arch anatomy.(14,16) This is the major obstacle to SENTINEL CPS positioning, as its design does not allow deployment from a left radial approach. Our experience with the SENTINEL CPS was similar, with no safety issues, a 95% deployment success and an additional time of approximately eight minutes. Importantly, we had only recently started to use the SENTINEL CPS, and with increasing experience, it is likely that the deployment success rate may improve and the additional time required will decrease.
Although the randomised trials of TAVI with the SENTINEL CPS did not show significant stroke reduction because they were underpowered,(12-14) larger pooled analysis and propensity-matched studies showed a reduction in periprocedural stroke from 4.6%–5.44% to 1.4%–1.88%, with cerebral protection.(15,16) The SENTINEL CPS would not be expected to completely eliminate 30-day stroke, as it can only prevent or reduce stroke during the immediate periprocedural period (< 24 hours after the procedure) and would not have any effect on the 45% of strokes that are known to occur subsequently.(8) However, any reduction in stroke burden is highly meaningful, as it adversely affects quality of life and increases mortality threefold.(8) The aforementioned studies, however, have not shed light on which patients are most likely to benefit from the use of a SENTINEL CPS during TAVI.
In our study, none of the patients developed a stroke. This compares favourably with recent real-world data, where the incidence of 30-day stroke was 2.3% in > 10,000 patients undergoing TAVI without cerebral protection(9) and 1%–2% in patients undergoing TAVI with the SENTINEL CPS.(15,16,25)
Cognitive decline has been shown to occur in 8%–12% of patients after TAVI,(11,23,27) with silent cerebral ischaemic lesions being strongly correlated with neurocognitive decline.(12,23) Cerebral protection with the SENTINEL CPS may reduce cognitive decline, although the current data is conflicting.(12,13) This may be due to the fact that up to a quarter of these silent cerebral ischaemic lesions were in the vertebrobasilar territory,(23) and some of these emboli via the left vertebral artery would not be protected with the SENTINEL CPS. In addition, up to 70%–80% of patients have complete resolution of these silent cerebral ischaemic lesions on MR imaging at 3–4 months.(22,23)
These studies used more extensive neurocognitive tests such as the Montreal Cognitive Assessment (MoCA) and the Mini-Mental State Examination (MMSE).(11,23) These tests, however, require trained staff to administer and can be time-consuming. The AMT is much simpler to perform, takes only a few minutes and has been validated against the MoCA and MMSE for detecting cognitive impairment.(19,28) The brevity of the AMT makes it more acceptable to patients and may reduce test error due to language barriers.(29) An AMT score cut-off of seven and below suggests abnormal cognitive function.(18,19) However, the AMT was developed mainly as a screening tool for cognitive impairment and was not intended to assess serial cognitive decline.
In this study, preservation of cognitive function was observed, with a similar baseline and 30-day mean AMT score. AMT score decreased by ≥ 2 in 10% of patients, although only 1 (2.5%) additional patient developed impaired cognition at 30 days compared with baseline (4 vs. 3, p = 1.0). A serial decrease in AMT score by ≥ 2 was determined to be significant in some studies(29,30) despite the lack of validation for AMT score in serial cognitive monitoring. Our results compare positively with a study showing that 25% of patients had a decreased neurocognitive test score, with 8% developing short-term abnormal cognition that persisted in 2% of patients at one year.(11) Cognitive decline has also been shown to occur in 30%–40% of patients at two months after cardiac surgery, falling to 10%–20% at one year.(31) Whether there might be similar improvements in our patients with longer follow-up is unknown. However, as we did not have a control arm without the use of the SENTINEL CPS, no firm conclusion on device efficacy in preservation of AMT scores can be drawn.
Conversely, in our study, 12.5% of patients showed an increase in AMT score, which has been hypothesised to be caused by augmented cerebral blood flow as a result of improved cardiac output after relief of the severe AS(11,27) or simply due to the ‘learning effect’.(24) This is consistent with previous reports showing that cognitive improvement occurred after TAVI in some patients, more noticeably in those with impaired baseline cognition.(11,27)
A striking finding in the current study was that patients who exhibited a decline in AMT score were significantly older, with none aged below 76 years. Age has been shown to be independently associated with cognitive decline after TAVI,(32) likely because older patients tend to have more age-related white matter changes (reduced cerebral reserve),(33) which increase the risk of silent cerebral ischaemic lesions and cognitive decline after TAVI.(23) This finding has implications for the counselling of elderly patients under consideration for TAVI; even if stroke risk is negligible, they may be at a higher risk of cognitive decline.
Our study was not without limitations. It was a single-centre feasibility study with a limited number of patients and only an intervention arm; hence, the data should be interpreted with caution. There was no independent neurology assessment, and we cannot exclude the possibility that minor strokes may have been missed, which could have affected the results of this study. The zero-stroke rate could also have been due to the newer-generation TAVI devices used, the younger mean age (and lower-risk profile) of our study population and an element of chance owing to the limited sample size. These factors may also have contributed to the good clinical outcomes (e.g. no 30-day mortality, very low permanent pacemaker rate). The neurocognitive assessment was not blinded, and investigator bias could not be excluded. The AMT is also a simple screening tool for the presence of impaired cognition and lacks sensitivity or specificity for the detection of serial cognitive deterioration. There was also no control group without the use of the SENTINEL CPS, and hence, no definite conclusions can be drawn on the efficacy of the device in stroke reduction or cognitive preservation.
In conclusion, we report the initial experience of TAVI with cerebral protection using the SENTINEL CPS in Southeast Asia. The use of Sentinel CPS was safe, with a high rate of successful deployment and required approximately eight minutes of additional procedure time. There was no 30-day stroke or death, and overall cognition was preserved. Only 1 (2.5%) patient developed cognitive impairment at 30 days. Larger studies will be required to further evaluate the role of the SENTINEL CPS during TAVI in Asian patients.
About the First Author

Dr Paul Chiam is a cardiologist subspecialising in interventional cardiology. He graduated on the Dean’s list from the National University of Singapore and was awarded the Gordon Ransome Gold Medal at the Master of Medicine (Internal Medicine) exam. He performed the first percutaneous transcatheter aortic valve replacement procedure in Asia and serves as a proctor to train new centres across Asia. He is trained in and performs complex coronary angioplasty, carotid and peripheral artery angioplasty and structural heart interventions. Dr Chiam is an Adjunct Associate Professor at the NUS Yong Loo Lin School of Medicine, Singapore, where he volunteers his time teaching medical students.


