Abstract
INTRODUCTION
Large-volume paracentesis (LVP) is the first-line treatment for decompensated cirrhosis with refractory ascites. While ascitic drain removal (ADR) within 72 hours of the procedure was once considered safe, it was uncertain whether ADR within 24 hours could further reduce the risk of ascitic drain-related bacterial peritonitis (AdBP). This study aimed to investigate the association between the timing of ADR and the presence of AdBP.
METHODS
All patients with cirrhosis with refractory ascites who underwent LVP in our institution from 2014 to 2017 were studied. AdBP was diagnosed based on an ascitic fluid neutrophil count ≥ 250 cells/mm3 or positive ascitic fluid culture following recent paracentesis within two weeks.
RESULTS
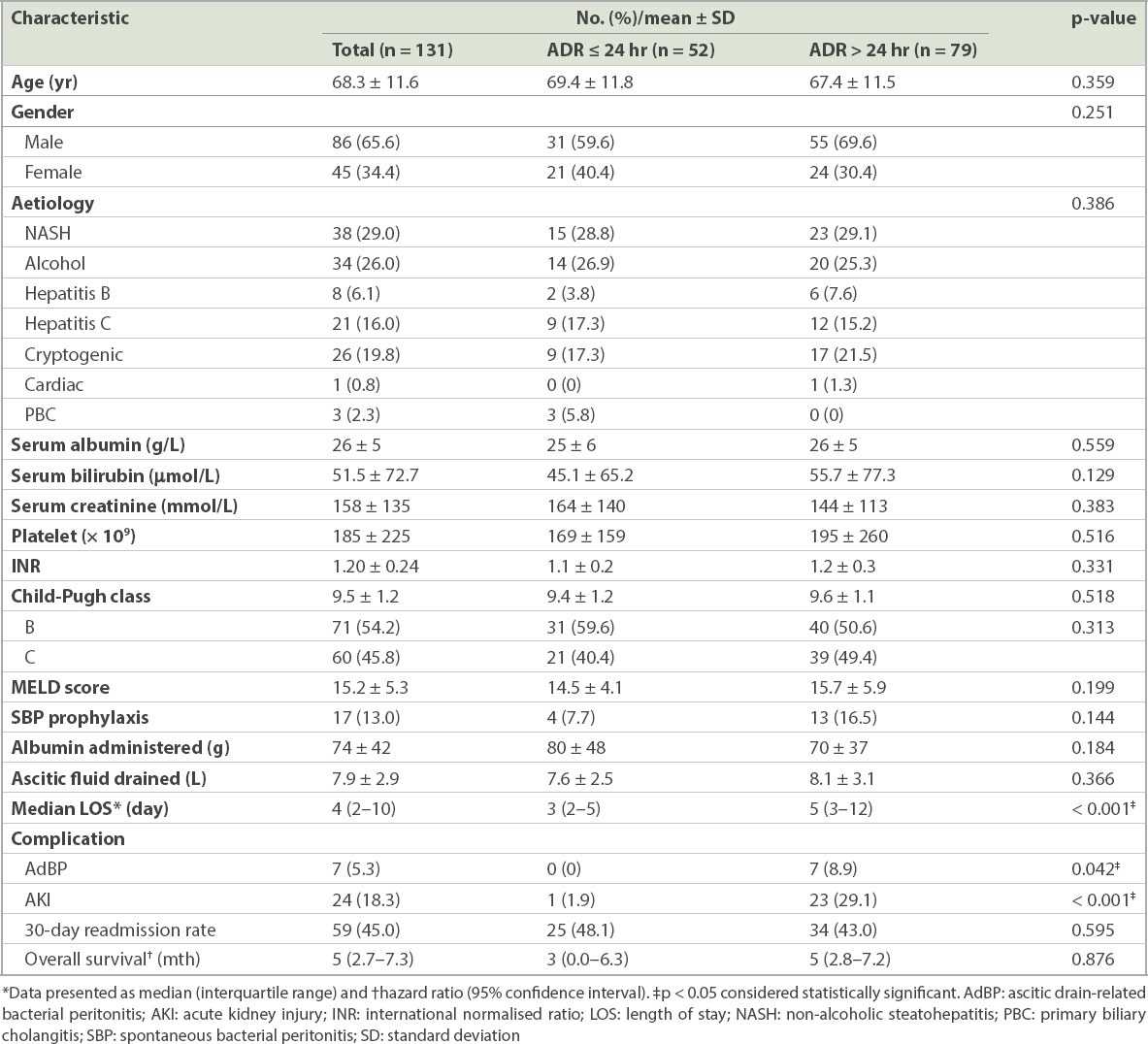
A total of 131 patients who underwent LVP were followed up for 1,806 patient-months. Their mean age was 68.3 ± 11.6 years, and 65.6% were male. Their mean Model for End-Stage Liver Disease score was 15.2. The overall incidence of AdBP was 5.3%. ADR beyond 24 hours was significantly associated with a longer median length of stay (five days vs. three days, p < 0.001), higher risk of AdBP (0% vs. 8.9%, p = 0.042) and acute kidney injury (AKI) following LVP (odds ratio 20.0, 95% confidence interval 2.4–164.2, p = 0.021). The overall survival was similar in patients who underwent ADR within and beyond 24 hours of LVP.
CONCLUSION
ADR within 24 hours of LVP is associated with a reduced risk of AdBP and AKI. As AdBP is associated with resistant organisms and AKI, we recommend prompt ADR within 24 hours, especially in patients who have Child-Pugh class C alcoholic cirrhosis.
INTRODUCTION
Refractory ascites, a common complication among patients with decompensated cirrhosis, is associated with poor survival and significantly impaired quality of life from abdominal bloating, immobility, development of hernia and sarcopenia.(1) Refractory ascites is defined as ascites that cannot be mobilised, either owing to diuretic-induced complications (diuretic-intractable ascites) or a lack of response to diuretics (diuretic-resistant ascites).(2) While liver transplantation is curative, the high cost of organ transplantation and organ scarcity limits access for patients with refractory ascites. The majority of cirrhosis patients with refractory ascites are, thus, dependent on palliative options such as large-volume paracentesis (LVP) or transjugular intrahepatic portosystemic shunt for symptom relief. Current guidelines by the American Association for the Study of Liver Diseases and European Association for the Study of the Liver (EASL) recommend LVP as the first-line treatment for patients with refractory ascites.(2,3)
Although LVP is an effective treatment option for cirrhosis patients with refractory ascites and is associated with lower readmission risk when compared to diuretics,(4) complications such as paracentesis-induced circulatory dysfunction (PICD), acute kidney injury (AKI) and ascitic drain-related bacterial peritonitis (AdBP) can occur with LVP.(5-7) For instance, PICD can occur in up to 75% of cirrhosis patients undergoing LVP as a result of haemodynamic changes following LVP in the setting of excessive systemic arterial vasodilatation. PICD has also been associated with rapid re-accumulation of ascitic fluid, AKI and hepatorenal syndrome. The development of AKI was shown to adversely affect the outcome of cirrhosis patients, resulting in extended hospitalisation, increased inpatient stays and increased 90-day mortality. Current evidence indicates that intravenous albumin is effective in preventing PICD, especially when ascitic drainage greater than 5 L is performed.(6,8,9) A slower rate and small amount of ascitic drainage was also shown to reduce the risk of PICD and AKI.(10-13)
To prevent PICD and AKI following LVP, some physicians may allow slower ascitic drainage over a more extended period. While delayed ascitic drain removal (ADR) can theoretically increase the risk of AdBP, ADR within 72 hours was previously considered safe among patients with decompensated cirrhosis.(14,15) Kathpalia et al reported a higher risk of AdBP and poorer survival in patients who underwent ADR after 72 hours of LVP.(14) However, the literature on the association between the timing of ADR and the occurrence of AdBP remains limited. It is unclear whether ADR within 24 hours of LVP, when compared to ADR beyond 24 hours, could further reduce the risk of AdBP without increasing the risk of AKI or readmission rates. Hence, this study aimed to determine the association between the timing of ADR and AdBP as well as the clinical impact of AdBP among cirrhosis patients with refractory ascites. We hypothesised that ADR within 24 hours of LVP could further reduce the risk of AdBP as compared to ADR beyond 24 hours.
METHODS
This was a retrospective study conducted in Changi General Hospital, a 1,000-bed teaching hospital serving a population of 1.3 million from the eastern and northeast regions of Singapore. All hospitalised patients with decompensated cirrhosis who had undergone LVP in Changi General Hospital from January 2014 to December 2017 were included.
Patients were first identified from electronic medical records using the procedure code for paracentesis and ICD-9 (International Classification of Diseases, ninth revision) codes for liver cirrhosis and ascites. To ensure the accuracy and consistency of our data, two authors (WYJ and LHM) separately reviewed the electronic medical records of all patients as well as the relevant data on patients’ baseline characteristics, laboratory results, clinical outcomes, and duration and complications of LVP. Cases were censored at death, liver transplant or last follow-up. A standard dose of albumin infusion was administered during LVP, as per EASL guidelines. Our institutional review board approved the study protocol.
All adults (age ≥ 21 years) who were diagnosed with liver cirrhosis and underwent paracentesis were consecutively included. The exclusion criteria were: (a) patients with spontaneous bacterial peritonitis (SBP) diagnosed upon admission or during insertion of a peritoneal drain; (b) patients with ascites secondary to malignancy, pancreatitis, nephrotic syndrome, non-cirrhotic portal hypertension and infection, including tuberculous peritonitis; and (c) patients who had undergone a diagnostic abdominal tap. After excluding patients with missing data, we identified a total of 131 patients with refractory ascites who had undergone LVP for analysis.
Cirrhosis was defined based on clinical, biochemical, radiological or histological findings. Non-alcoholic steatohepatitis (NASH) was defined as cirrhosis with metabolic syndrome after exclusion of other liver diseases such as chronic viral hepatitis, autoimmune liver disease and Wilson’s disease. LVP was defined as ascitic drainage to relieve symptoms arising from refractory ascites.(16) AdBP was diagnosed based on ascitic fluid analysis, especially a neutrophil count ≥ 250 cells/mm3 or positive ascitic fluid culture following recent paracentesis within two weeks. AKI was defined as an increase in serum creatinine of > 26.5 mmol/L from baseline within 48 hours following LVP.(2) For the diagnosis of alcoholic liver cirrhosis, clinically significant alcohol intake was defined as alcohol consumption of > 20 g/day for women and > 30 g/day for men.(17)
Statistical analysis was conducted using IBM SPSS Statistics version 22.0 (IBM Corp, Armonk, NY, USA). Descriptive statistics were computed for all variables. Categorical variables were expressed as frequencies and percentages, while mean and standard deviation were expressed as continuous variables. Categorical variables were analysed using chi-square or Fisher’s exact test. Continuous variables were analysed using Student’s t-test. All p-values quoted were two-sided, and p < 0.05 was considered statistically significant. ADR within 24 hours was the dependant variable. Binary logistic regression was performed by adjusting for covariates that were clinically relevant to AdBP. Cox proportional hazards regression was used for mortality analysis.
RESULTS
The analysis included 131 patients with cirrhosis who had undergone LVP. The mean age of the patients was 68.3 ± 11.6 years. The mean Model for End-Stage Liver Disease (MELD) score was 15.2 ± 5.3 years. The commonest cause of underlying liver cirrhosis was NASH (29.0%), followed by alcohol (26.0%), cryptogenic (19.8%) and chronic hepatitis C (16.0%). A total of 17 (13.0%) patients received antibiotics for secondary prophylaxis while undergoing LVP. The median duration of ADR was 2 (interquartile range 2–10) days, and 65.6% of the patients underwent ADR within two days.
The baseline demographics of patients who underwent ADR within and beyond 24 hours of LVP are summarised in
Table I
Baseline demographics of patients with decompensated cirrhosis who underwent ascitic drain removal (ADR) within and beyond 24 hours of large-volume paracentesis.
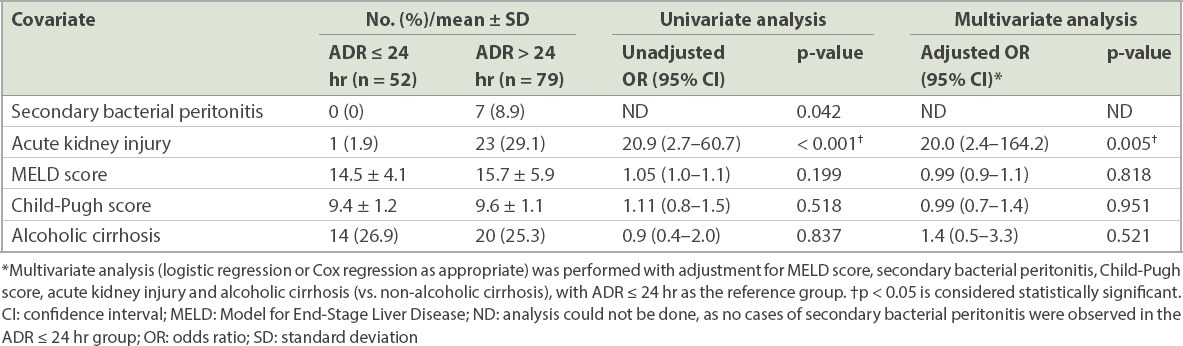
ADR beyond 24 hours of LVP was associated with a higher risk of AKI (odds ratio [OR] 20.0, 95% confidence interval [CI] 2.4–164.2; p = 0.005) after adjusting for MELD score, Child-Pugh score, AdBP and alcoholic liver cirrhosis (
Table II
Association between clinical outcomes and ascitic drain removal (ADR) within and beyond 24 hours of large-volume paracentesis.
The overall incidence of AdBP in our cohort was 5.3% (7/131). The baseline characteristics of patients with and without AdBP are summarised in
Table III
Baseline demographics of patients with and without ascitic drain-related bacterial peritonitis (n = 131).
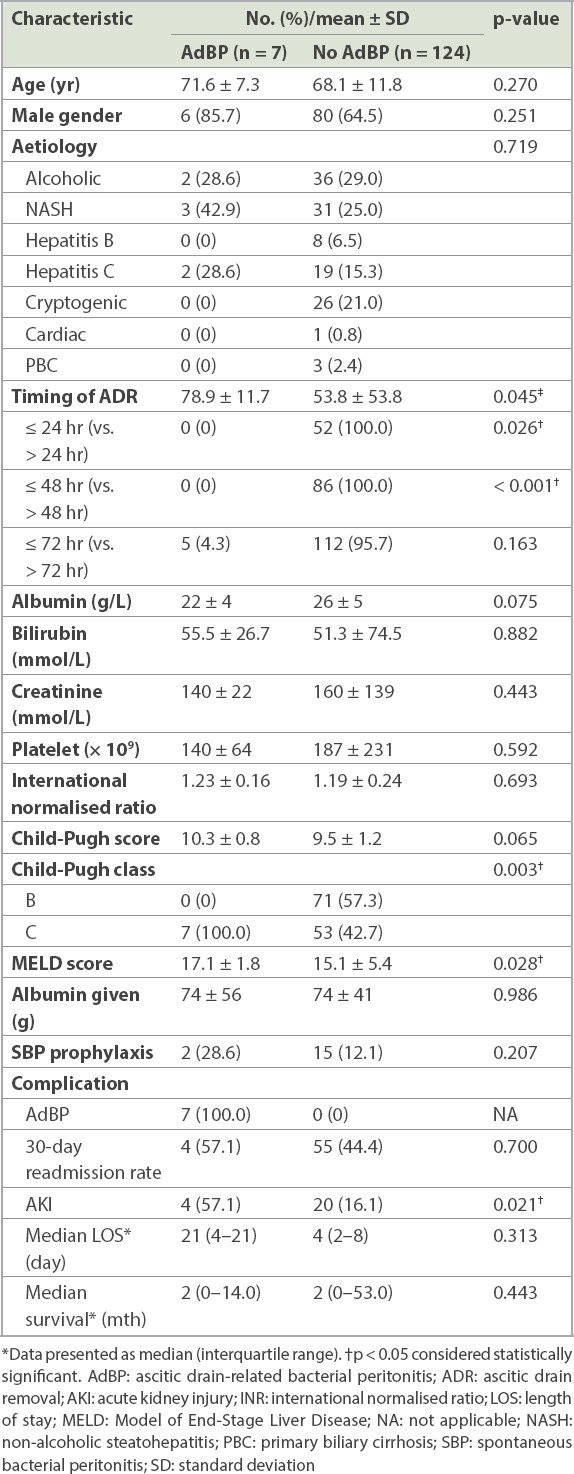
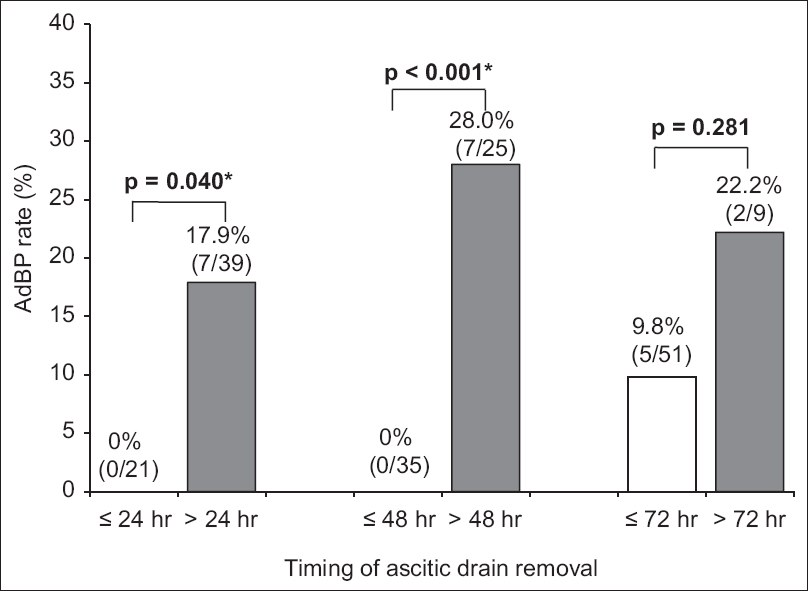
Fig. 1
Chart shows the rates of ascitic drain-related bacterial peritonitis and the timing of ascitic drain removal in all patients. *p < 0.05 is considered statistically significant. AdBP: ascitic drain-related bacterial peritonitis
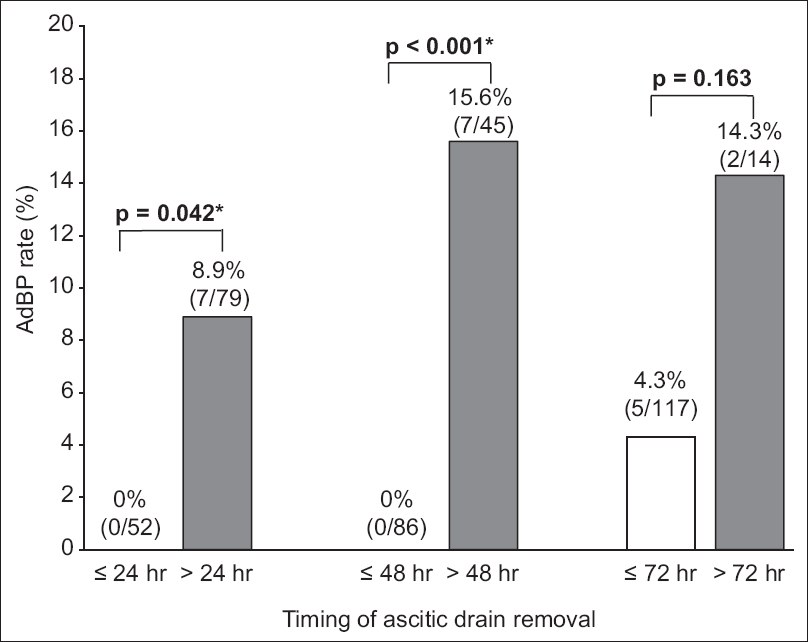
Fig. 2
Chart shows the rates of ascitic drain-related bacterial peritonitis and the timing of ascitic drain removal in patients with Child-Pugh class C cirrhosis. AdBP: ascitic drain-related bacterial peritonitis
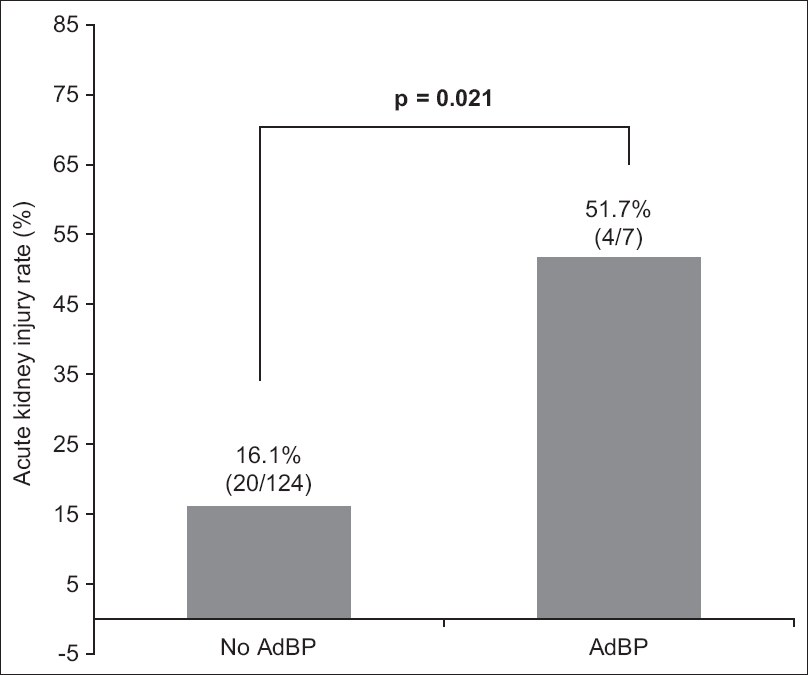
AdBP was also associated with a higher risk of AKI following LVP (57.1% vs. 16.1%, p = 0.021) (
Fig. 3
Chart shows the rates of acute kidney injury with and without ascitic drain-related bacterial peritonitis. AdBP: ascitic drain-related bacterial peritonitis
Among all patients with AdBP, 71.4% (5/7) had resistant organisms from ascitic fluid cultures (Extended-spectrum beta-lactamases-producing Escherichia coli: n = 3, Methicillin-resistant Staphylococcus aureus: n = 2, Bacteroides ovatus: n = 2). The proportion of patients receiving SBP prophylaxis was similar among patients with and without AdBP (28.6% vs. 12.2%, p = 0.207).
DISCUSSION
This study demonstrates that ADR beyond 24 hours of LVP increases the risk of AdBP and AKI in patients undergoing LVP. While none of the patients who underwent ADR within 24 hours had AdBP, ADR beyond 24 hours and 48 hours was associated with a significantly higher risk of AdBP (8.9% and 15.6%, respectively) (
AdBP has important clinical implications among patients undergoing LVP. AdBP is not only preventable but is also associated with a higher risk of AKI and prolonged hospitalisation, particularly among cirrhosis patients with higher MELD scores. Prolonged hospitalisation, in turn, increases the direct healthcare costs for patients and predisposes them to a higher risk of nosocomial infections by multidrug-resistant organisms (MDROs).(19) The emergence of MDROs in AdBP significantly impacts the survival of hospitalised cirrhosis patients and is associated with a higher risk of acute-on-chronic liver failure (ACLF). It is now clear that ACLF carries a high 90-day mortality of 50.4%–56.1% among hospitalised patients with decompensated cirrhosis.(17,20) As a palliative procedure, the goal of LVP should be symptom relief rather than complete drainage of ascites. Our data supports prompt ADR within 24 hours to mitigate the risk of AdBP and AKI from LVP.
Despite a higher risk of AdBP and AKI, overall survival was similar between patients who underwent ADR within 24 hours and beyond 24 hours of LVP, after adjusting for various clinically relevant confounding factors. Meanwhile, patients with alcoholic cirrhosis had a two-fold higher risk of mortality as compared to patients with non-alcoholic cirrhosis (HR 2.2, 95% CI 1.4–3.5, p = 0.002). The association between alcoholic cirrhosis and a higher incidence of ascites and SBP has been reported in previous studies.(21,22) Postulated mechanisms of these findings included increased gut permeability, impaired immunity and gut dysbiosis.(23-25) Alcohol abstinence may improve liver function, thus potentially improving transplant-free survival among alcoholic cirrhosis patients with ascites.(26) Therefore, physicians should be vigilant in performing prompt ADR among alcoholic cirrhosis patients who require LVP.
We acknowledge that this study must be interpreted within its limitations. Firstly, our study had a retrospective and non-randomised design. Owing to the small number of events, logistic regression analysis to assess the OR of AdBP could not be conducted. The small number of events also precluded multivariate analysis for confounders of AdBP. As all the events were observed in patients with Child-Pugh Class C cirrhosis, it is conceivable that the severity of liver disease is a significant contributor to the development of AdBP. A subgroup analysis within patients with Child-Pugh Class C cirrhosis showed that ADR within 24 hours of LVP remained significantly associated with AdBP (
The results of this study should be widely applicable, because we included all patients with decompensated liver cirrhosis with refractory ascites undergoing LVP, regardless of the severity of the liver cirrhosis and the underlying aetiology. The baseline characteristics were also comparable between the patients who underwent ADR within and beyond 24 hours of LVP. Further, ADR beyond 24 hours of LVP was significantly associated with a higher risk of AdBP and AKI, even after adjusting for clinically significant confounding factors.
In conclusion, ADR beyond 24 hours of LVP increases the risk of AKI and AdBP, and is linked to a significant risk of AdBP. As AdBP is associated with more resistant organisms and a higher risk of AKI, prompt ADR within 24 hours is recommended, especially among patients with alcoholic cirrhosis with Child-Pugh Class C or a higher MELD score. While it is unlikely that a prospective study will be performed, these findings should serve as a reminder to clinicians that leaving a drain for longer durations increases the risk of AdBP and that there is no merit in protracted ascitic drainage.
About the First Author

Dr Wong Yu Jun is a Clinician Scientist and Consultant gastroenterologist at Changi General Hospital, Singapore. He is passionate about management of advanced liver disease, particularly in the management of portal hypertension-related complications in advanced cirrhosis patients. He is also interested in microelimination of chronic hepatitis C, particularly among the incarcerated population. He is a recipient of the Nurturing Clinician Scientist Award from the SingHealth Duke-NUS Medicine Academic Clinical Programme and a Master of Clinical Investigation scholar, National Medical Research Council, Singapore.


