Abstract
INTRODUCTION
There are concerns that angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) may worsen the outcomes of patients with COVID-19. This systematic review and meta-analysis aimed to study the in-hospital mortality among COVID-19 patients who were on ACEIs/ARBs as compared to those not on ACEIs/ARBs.
METHODS
We searched PubMed, EMBASE, clinicaltrials.gov and Google Scholar between 1 January 2020 and 30 May 2020 to identify all studies that evaluated the use of ACEIs/ARBs and reported the in-hospital mortality outcomes of COVID-19 patients. Nine non-randomised studies were eligible for inclusion in the analysis. The primary outcome studied was the in-hospital mortality of COVID-19 patients who were on ACEIs/ARBs compared with those not on ACEIs/ARBs.
RESULTS
Of the 8,313 patients in the nine studies, 7,622 (91.7%) were from studies with all-comers, while 691 (8.3%) were from studies involving only patients with hypertension. 577 (14.6%) in-hospital deaths were observed out of a total of 3,949 patients with an outcome in the nine studies. Overall, no significant difference was observed in the in-hospital mortality between patients on ACEIs/ARBs and those not on ACEIs/ARBs (odds ratio [OR] 1.06, 95% confidence interval [CI] 0.75–1.50; p = 0.73). Further sensitivity analysis in the hypertension group and the all-comers group showed similar results (OR 0.88, 95% CI 0.58–1.32; p = 0.53 and OR 1.85, 95% CI 1.00–3.43; p = 0.05, respectively).
CONCLUSION
We observed that ACEIs/ARBs had no significant impact on the in-hospital mortality of COVID-19 patients and can be used safely in patients with indications.
INTRODUCTION
COVID-19 is a global pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first reported in Wuhan, Hubei, China, in December 2019.(1) It has since posed a serious socioeconomic burden and put an unprecedented strain on healthcare systems worldwide. Hypertension and cardiovascular disease are common morbidities among patients with COVID-19, and they have also been shown to be associated with higher mortality and worse outcomes.(2-5)
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are renin-angiotensin-aldosterone system (RAAS) inhibitors that are commonly prescribed for hypertension, cardiac disease and renal disease. The use of RAAS inhibitors has been reported to increase the expression of angiotensin-converting enzyme 2 (ACE2) in animal studies.(6) As ACE2 serves as the receptor in SARS-CoV-2 for host cell entry, there have been concerns that a higher ACE2 expression may lead to worse outcomes in patients with COVID-19 who are taking ACEIs/ARBs.(7-9) Multiple major international societies have since published position statements regarding the use of ACEIs/ARBs in patients with COVID-19, recommending the continuation of these medications for patients who have clinical indications such as heart failure and hypertension.(10,11)
The significance of ACEIs/ARBs in the clinical outcomes of patients with COVID-19 has mostly been studied in small observational studies on these patients. This systematic review and meta-analysis sought to quantify the effect of ACEIs/ARBs on the risks of in-hospital mortality in patients with COVID-19.
METHODS
This meta-analysis was performed in adherence to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and MOOSE (Meta-analysis of Observational Studies in Epidemiology) checklist on the quality of reporting of meta-analyses.(12) The study was approved by the SingHealth Centralised Institutional Review Board (2020/2452).
Two independent reviewers (VHT and XJC) searched PubMed, EMBASE, clinicaltrials.gov and Google Scholar for relevant articles on ACEI/ARB-related outcomes in patients with COVID-19. The following search terms were used in the literature review: ‘COVID-19’, ‘coronavirus disease 2019’, ‘angiotensin-converting enzyme inhibitors’, ‘ACE inhibitors’, ‘ACEI’, ‘angiotensin receptor blockers’, ‘ARB’, ‘renin angiotensin aldosterone system inhibitors’ and ‘renin angiotensin aldosterone system’. The reference lists of all published studies and biographies of review articles were also searched to identify additional articles. No language or publication status restrictions were applied. The search was conducted from 1 January 2020 (as COVID-19 was first reported in late December 2020) to 30 May 2020. Only studies in which in-hospital mortality data was reported or available were included. The corresponding authors of the studies were also contacted to provide their unpublished data. Any disagreements were resolved in a panel discussion consisting of three reviewers (VHT, JCKT and XJC).
Study selection involved the screening of titles and abstracts, followed by full-text evaluation of the eligible studies. The inclusion criteria were: (a) study population comprising all patients with COVID-19 with or without ACEI/ARB use and (b) reported in-hospital mortality outcomes. The primary outcome measure was in-hospital mortality. Data was independently extracted by two of the study investigators (VHT and JCKT) using a standardised protocol and reporting form (Appendix). Any disagreements were resolved by arbitration, and consensus was achieved after discussion. The following data was collected: study characteristics (study name, authors, sample size, study design and follow-up duration); study sample characteristics (mean age, gender, major comorbidities and prescription of ACEI/ARB); and main outcomes.
Data was pooled and analysed using RevMan Version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014). A random effects model was used for summarising effects. The effect size is presented as odds ratio (OR) with 95% confidence interval (CI). All statistical tests were two-sided and used a significance level of p < 0.05. Statistical heterogeneity was evaluated using the Higgins and Thompson I2 statistic. The I2 is the proportion of total variation observed among the studies that is attributable to differences between studies rather than sampling error (chance), with I2 values corresponding to the following levels of heterogeneity: low (< 25%); moderate (25%–75%); and high (> 75%).(13) Reasons for the heterogeneity in the study results were further explored using subgroup analyses, which were performed according to the presence of hypertension as a comorbid. We also performed a sensitivity analysis to investigate the association of each individual study with the overall meta-analysis results.
RESULTS
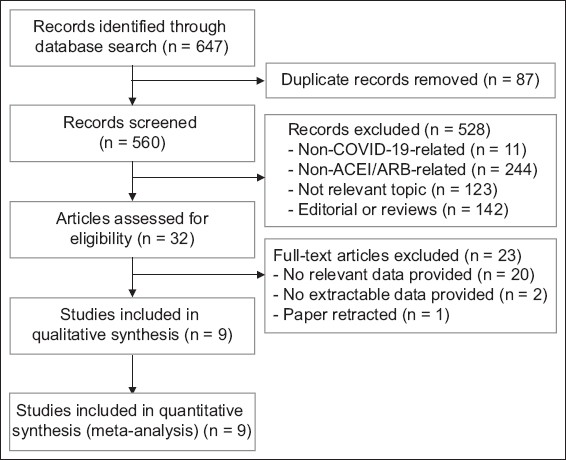
Our database search retrieved 560 unique studies, 528 of which were deemed irrelevant based on title and abstract screenings. The full text of 32 studies was assessed for eligibility, of which nine met the final inclusion criteria and were included in the main analysis of this study (
Fig. 1
Flowchart shows the study selection process. ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers
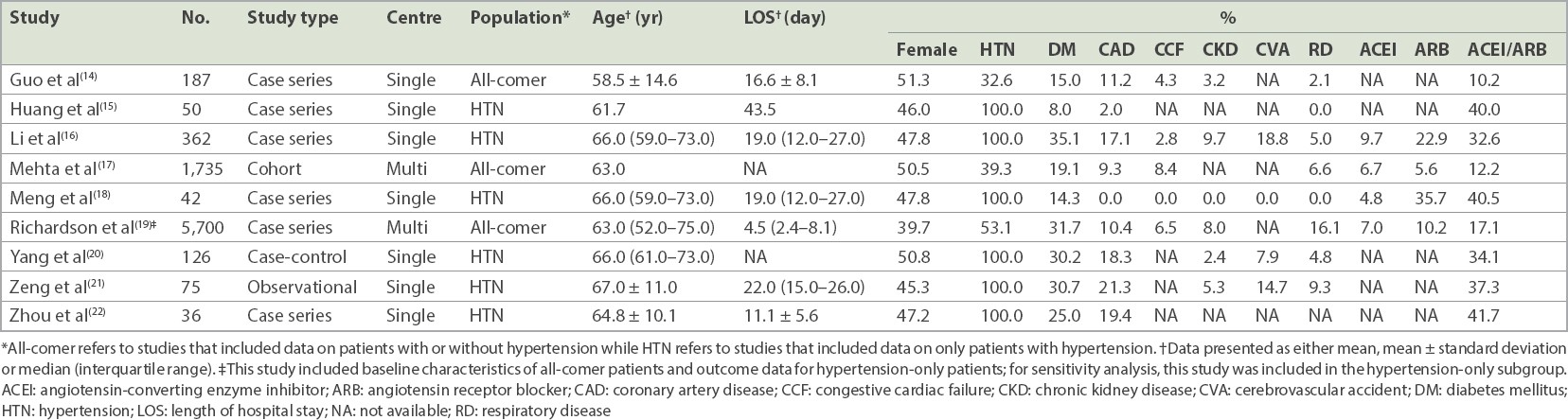
The characteristics of the patients in these nine studies are shown in
Table I
Characteristics of all patients included in the analysis.
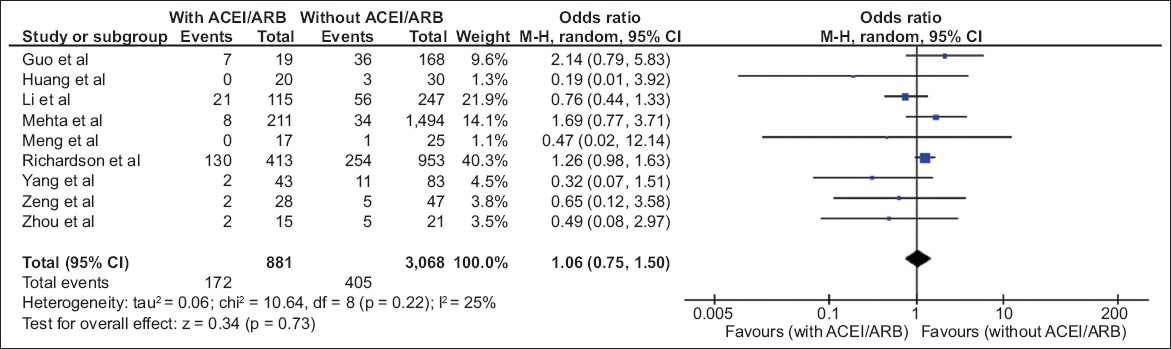
In terms of the use of ACEIs/ARBs, the hypertension-only group had, unsurprisingly, a higher proportion of ACEI/ARB use compared with the all-comers group (34.5% vs 5.7%). Out of the 3,949 patients with outcomes in the combined nine studies, 577 (14.6%) in-hospital deaths were observed, with 172 (4.4%) deaths in the ACEI/ARB group and 405 (10.2%) deaths in the non-ACEI/ARB group. No significant increase in in-hospital mortality was observed in the ACEI/ARB group versus the non-ACEI/ARB group (OR 1.06, 95% CI 0.75–1.50, p = 0.73;
Fig. 2
Diagram shows the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blocker (ACEIs/ARBs) vs. non-ACEI/ARB use in all patients with COVID-19 and the risk of in-hospital mortality (events) for the nine included studies.(14-22) CI: confidence interval; M-H: Mantel-Haenszel test
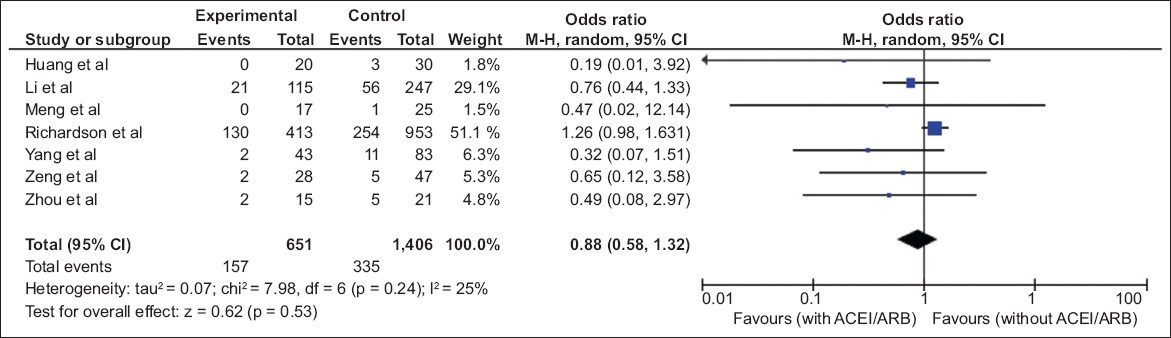
In total, seven studies with in-hospital mortality outcomes focused on patients with hypertension alone.(15,16,18-22) One study by Richardson et al(19) presented the clinical characteristics of all-comers but only reported in-hospital mortality for patients with hypertension. In this subset of 2,057 hypertension-only patients, there were 492 (23.9%) in-hospital deaths – 157 (7.6%) deaths in the ACEI/ARB group and 335 (16.3%) deaths in the non-ACEI/ARB group. Similarly, the use of ACEIs/ARBs in this study was not associated with any significant difference in in-hospital mortality in the hypertension-only group (OR 0.88, 95% CI 0.58–1.32, p = 0.53;
Fig. 3
Diagram shows the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blocker (ACEIs/ARBs) vs. non-ACEI/ARB use in patients with COVID-19 who had hypertension and the risk of in-hospital mortality (events) for the seven studies that reported in-hospital mortality in both subgroups.(15,16,18-22) CI: confidence interval; M-H: Mantel-Haenszel test
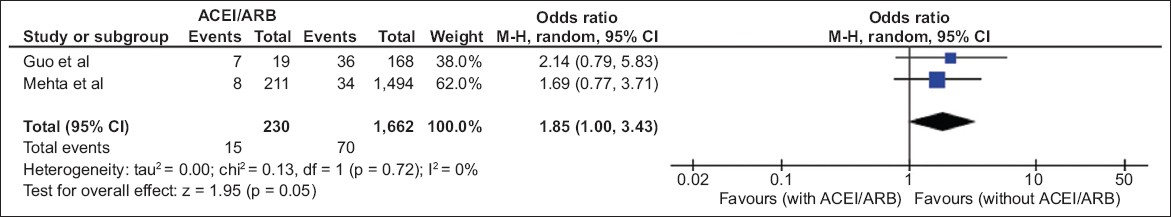
Two studies reported in-hospital mortality outcomes in all-comers.(14,17) In the subset of all-comers, 85 (4.5%) in-hospital deaths were reported out of 1,892 patients across the two studies – 15 (0.8%) deaths in the ACEI/ARB group and 70 (3.7%) deaths in the non-ACEI/ARB group. The use of ACEIs/ARBs was not associated with any significant difference in the in-hospital mortality in all-comers (OR 1.85, 95% CI 1.00–3.43, p = 0.05;
Fig. 4
Diagram shows the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blocker (ACEIs/ARBs) vs. non-ACEI/ARB use in all-comer patients with COVID-19 and the risk of in-hospital mortality (events) for two studies that reported in-hospital mortality in both subgroups.(14,17) CI: confidence interval; M-H: Mantel-Haenszel test
DISCUSSION
This analysis demonstrated that (a) the use of ACEIs/ARBs does not result in a significant increase in in-hospital mortality outcomes in patients with COVID-19 and (b) there is a higher prevalence of comorbidities, particularly hypertension, in patients with COVID-19.
Hypertension is one of the top causes of premature death globally, with an estimated prevalence of 31.1% among the worldwide adult population aged more than 20 years in 2010.(23) Hypertension has also been frequently reported as a major comorbidity in patients with COVID-19, and this is further supported by our pooled analysis of 8,313 patients with a prevalence of hypertension of 49.4%.(1–5)
There is an ongoing debate regarding the effect of ACEIs/ARBs on the outcomes of patients with COVID-19 and whether these medications should be discontinued. This underlying concern stems from both previous and recent studies that have demonstrated that ACE2 is the cellular entry point and mediator of infection and transmission in not only severe acute respiratory syndrome coronavirus (SARS-CoV), the culprit coronavirus in the 2003 SARS outbreak, but also SARS-CoV-2.(24-26) This is further supported by studies that showed a 79% similarity in the genomic sequence of SARS-CoV-2 and SARS-CoV.(27)
However, this concern regarding the use of ACEIs/ARBs in patients with COVID-19 has not been proven in clinical studies. At the same time, it has been postulated that ACEIs/ARBs may have a beneficial effect on patients with COVID-19, with evidence of downregulation of ACE2 expression by SARS-CoV-2.(28) It has been shown that RAAS activation plays an important role in acute respiratory distress syndrome (ARDS).(29) Kuba et al found that downregulation of ACE2 by SARS-CoV, the culprit coronavirus for the 2003 SARS outbreak, worsens lung injury in mice models.(30) This suggested that modulation of the RAAS system could improve outcomes in ARDS. Moreover, stopping essential medications that are guideline-recommended treatment for hypertension, heart failure and kidney failure, among other indications, can have deleterious effects on patients’ blood pressure and haemodynamics.
Our findings that ACEIs/ARBs have no significant impact on in-hospital mortality in patients with COVID-19 are in keeping with the positional statements of major international professional societies, such as the American College of Cardiology, American Heart Association, Heart Failure Society of America and European Society of Cardiology.(10,11)
The present study has some limitations. Individual patient data was not available, limiting our ability to look at specific patient characteristics, such as clinical indication for ACEIs/ARBs, age and other comorbidities of the patient population. In addition, the short study period and availability of many studies posed a degree of limitation in terms of quality assessment of the included studies. However, given the urgency and global scale of the pandemic, it is crucial to include all available data to provide a more complete assessment of the effect of ACEs/ARBs on COVID-19-related mortality. The degree of publication bias was also likely to be limited given the call for openness and transparency of all COVID-19-related information.
Another important limitation is that most of the studies did not describe in detail the initiation, continuation and cessation of ACEI/ARB during hospitalisation, with the exception of Richardson et al’s study.(19) The definition of ACEI/ARB exposure was also not clearly defined, and thus, it was unclear whether these medications were prescribed on admission or throughout the admission period; this is important, as it may potentially compound the effect of the study. Richardson et al reported that among patients taking ACEIs/ARBs at home, 48.1% and 50.1% continued taking an ACEI and ARB, respectively, while in hospital. Of the 953 patients with hypertension who were not prescribed an ACEI or ARB at home, 49 and 58 patients started an ACEI and ARB, respectively, during hospitalisation.(19)
As COVID-19 is an emerging novel viral infection, the number of observational studies is limited and the study size is generally small. There were seven small studies contributing fewer than 500 patients each. Two large studies accounted for 7,435 (89.4%) out of the total patient population of 8,313 patients analysed.(17,19) Although we cannot rule out the possibility of selection bias, it is unlikely to have substantially influenced the outcome, as our meta-analysis included studies from multiple countries (China, Italy, United Kingdom and United States of America) with varying patient populations across the world. This, in fact, helps to strengthen the international societies’ position statements and reassure physicians managing patients with COVID-19 around the world.
The studies included in this meta-analysis are all either case-control or case-series observational studies, which have weaknesses that are inherent to observational data. To our knowledge, there are ongoing randomised clinical trials studying the use of ACEIs/ARBs in COVID-19, including one on Losartan, which will further clarify the effect of ACEIs/ARBs in COVID-19 mortality outcomes.(31)
In conclusion, this meta-analysis shows that ACEIs/ARBs have no significant impact on in-hospital mortality in patients with COVID-19. This result concurs with the recommendations made by major international professional societies to not discontinue ACEIs/ARBs in patients with relevant indications. This result will help to guide physicians caring for patients with COVID-19 on these medications.
SUPPLEMENTARY MATERIAL
The Appendix is available online at https://doi.org/10.11622/smedj.2020159.


