Abstract
Transvenous implantable cardioverter defibrillators are a type of implantable cardiac device. They are effective at reducing total and arrhythmic mortality in patients at risk of sudden cardiac death. Subcutaneous implantable cardioverter defibrillators (S-ICDs) are a new alternative that avoids the disadvantages of transvenous lead placement. In this case series, we report on the initial feasibility and safety of S-ICD implantation in Singapore.
INTRODUCTION
Implantable cardioverter defibrillators (ICDs) were first introduced into clinical practice in 1980.(1) Since then, many randomised multicentre trials have shown that ICDs are able to significantly reduce total and arrhythmic mortality in patients at risk of sudden cardiac death (SCD).(2-4) However, ICDs are also associated with significant complications.(5-9) Many of these complications arise due to the need for one or more transvenous leads. Acute, procedure-related complications include pneumothorax, cardiac perforation and tamponade, pericardial effusion and acute lead dislodgement. Longer-term complications include lead failure (which is commonly due to insulation breaks or conductor coil fractures, often leading to inappropriate shock therapy) and infection at the site of the lead. The latter may progress to native valve endocarditis, a condition that frequently necessitates lead extraction, a procedure that has been associated with significant morbidity and mortality.
Recently, an entirely subcutaneous ICD (S-ICD) system (i.e. the S-ICD™ System; Boston Scientific, Malborough, MA, USA) has entered clinical practice.(10-12) This S-ICD system senses, detects and treats ventricular tachycardia (VT) and ventricular fibrillation (VF) without the use of a transvenous lead. The subcutaneous pulse generator and electrodes are placed in an extrathoracic position. By eliminating the need for a transvenous lead, this system avoids most of the risks associated with a system that utilises an intravascular location. The main disadvantage of the S-ICD is that painless antitachycardia pacing cannot be delivered. The use of S-ICDs is relatively new in Asia. In this case series, we report on the initial feasibility and safety of S-ICD implantation in Singapore.
CASE SERIES
Five patients underwent S-ICD implantation at the National Heart Centre, Singapore, between March 2014 and June 2014.
Case 1
Patient 1 was a 55-year-old Chinese man who was categorised as New York Heart Association (NYHA) functional class II. He had a history of stable ischaemic heart disease that was associated with a severely depressed left ventricular ejection fraction (LVEF) of 25%. The patient had significant comorbidities, including end-stage renal failure (for which he was established on haemodialysis via a left radiocephalic arteriovenous fistula), hypertension, peripheral artery disease and infrarenal aortic aneurysm. A right pectoral transvenous ICD implantation was scheduled for primary prevention of SCD. However, as a right upper limb venogram showed a totally occluded right subclavian vein, transvenous ICD implantation was abandoned. The patient was subsequently counselled for S-ICD implantation.
Case 2
Patient 2 was a 56-year-old Chinese man in NYHA functional class II. He had stable non-ischaemic cardiomyopathy and an LVEF of 17%. His significant comorbidities included end-stage renal failure due to diabetic nephropathy, diabetic retinopathy, dyslipidaemia and previous cerebrovascular accident (with good functional recovery). He was established on haemodialysis via a left radiocephalic arteriovenous fistula. As his preprocedural venogram demonstrated subtotal occlusion of the right subclavian vein, the patient was referred for S-ICD implantation for primary prevention of SCD.
Case 3
Patient 3 was a 63-year-old Indian man in NYHA functional class II. He had stable ischaemic cardiomyopathy with an LVEF of 25%. His significant comorbidities included end-stage renal failure, type 2 diabetes mellitus, hypertension and dyslipidaemia. Renal replacement therapy was administered in the form of haemodialysis via a right-sided tunnelled dialysis catheter. He was referred for S-ICD implantation for primary prevention of SCD because some episodes of asymptomatic, nonsustained VT were noted during a ward admission.
Case 4
Patient 4 was a 39-year-old Malay man who was admitted with multiple episodes of unexplained syncope. Physical examination was unremarkable. Although the patient had no family history of SCD, he had Brugada syndrome. Echocardiography showed a structurally normal heart and exercise treadmill showed no inducible ischaemia or arrhythmias at good workload. He underwent a flecainide challenge test, which returned a positive result. After appropriate counselling, he opted for S-ICD implantation for primary prevention of SCD.
Case 5
Patient 5 was a 38-year-old Chinese man who was incidentally found to have Brugada syndrome. He admitted to occasional episodes of giddiness, without actual syncope. He underwent a flecainide challenge test, which returned a positive result. A treadmill exercise test showed that the patient was negative for ischaemia but had Brugada syndrome. An electrophysiology study for inducible VF was not performed. He had no family history of SCD. After appropriate counselling, he opted for S-ICD implantation for primary prevention of SCD.
Preimplant procedure
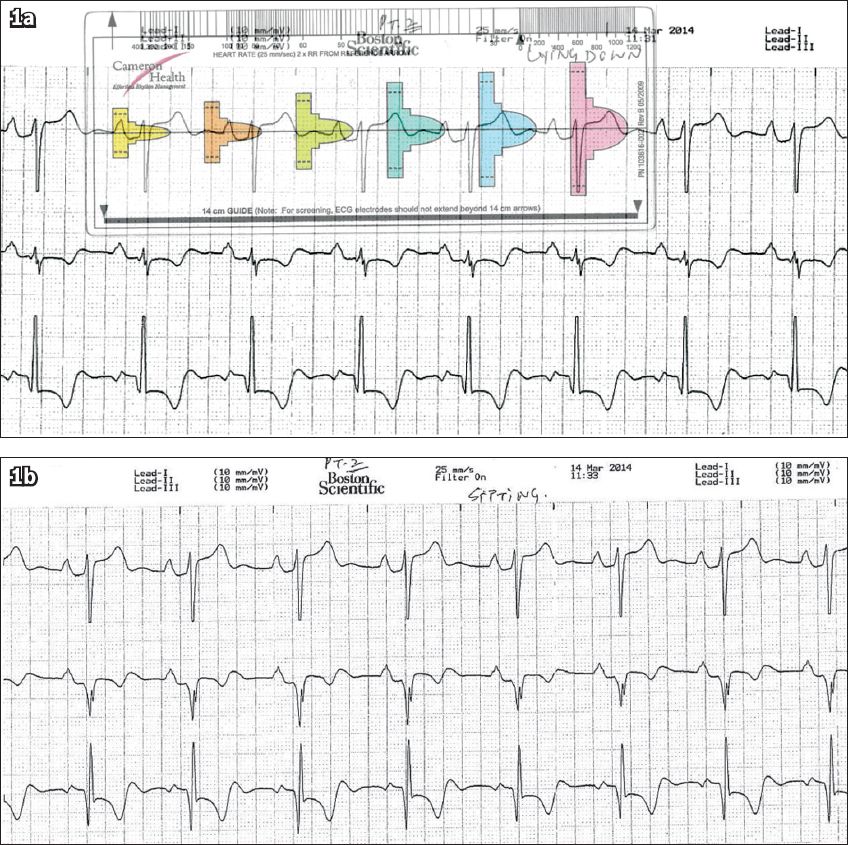
All patients underwent a modified screening ECG to confirm their suitability for S-ICD implant. The screening electrocardiograms were taken in at least two postures – lying down and sitting (
Fig. 1
Patient 1: Screening ECGs in the (a) lying down position and (b) sitting position. The QRS complex in (a) fits the pink template, confirming the patient’s suitability for subcutaneous implantable cardioverter defibrillator implantation.
Implant procedure
The patients were brought to the electrophysiology lab in the postabsorptive state. Conscious sedation was performed using a combination of midazolam and/or fentanyl boluses and/or propofol infusion. Using a dummy device as a guide, the position of the subcutaneous pocket was first marked over the sixth rib, between the left mid-axillary and anterior axillary lines. A horizontal line from this marking was then drawn to the left parasternal area, at the level of the lower border of the xiphoid. A vertical left parasternal line was then drawn from this point upwards. The appropriateness of these surface markings were confirmed using on-table fluoroscopy.
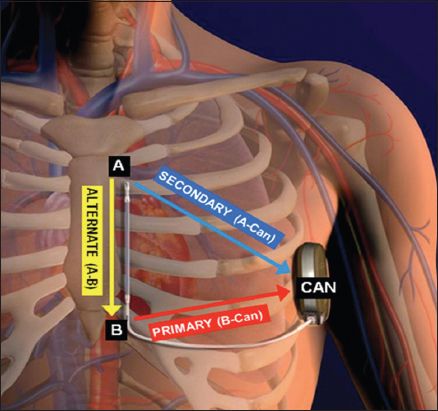
Fig. 2
Illustration shows the correct positioning of the pulse generator and electrode of the subcutaneous implantable cardioverter defibrillator (S-ICD). The S-ICD pulse generator should be just lateral to the cardiac apex, in the mid-axillary line. A: distal electrode ring; B: proximal electrode ring; CAN: pulse generator. (Illustration used with permission from Boston Scientific.)
The patient was then draped and prepared in a sterile way. The subcutaneous pocket at the lateral chest wall was fashioned following the defined surface markings. After the lower parasternal incision was made, a subcutaneous tunnel was created from the lower paraternal incision to the lateral chest wall pocket using a tunnelling tool. The S-ICD lead was then pulled through the established tunnel from the lateral chest wall pocket to the lower parasternal incision. The lead was then anchored to the underlying fascia using a suture sleeve 1 cm proximal to the sensing electrode ring. Another left parastenal incision was made at the second intercostal space. The rest of the lead was then directed superiorly (using the tunnelling tool), stretched out and finally secured at its distal end to the underlying fascia. The S-ICD lead was then connected to the pulse generator. This was placed in the lateral chest wall pocket. All pocket and skin incisions were closed in layers with absorbable sutures. Once the pocket and skin incisions were closed, a device check was performed. The optimal sensing vector was then determined using the automated S-ICD algorithm, following which defibrillation testing would be performed. Under conscious sedation, VF was induced using 50 Hz alternating current (AC) stimulation and defibrillation was tested at 65 J (
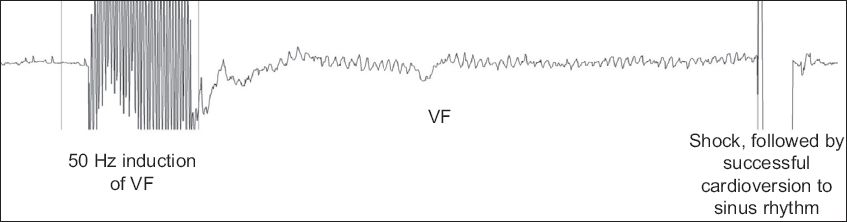
Fig. 3
Tracing during defibrillation threshold testing shows successful defibrillation following induction of ventricular fibrillation (VF) using 50 Hz alternating current stimulation.
All patients had S-ICD interrogation within 24 hours after implantation, along with chest radiography (
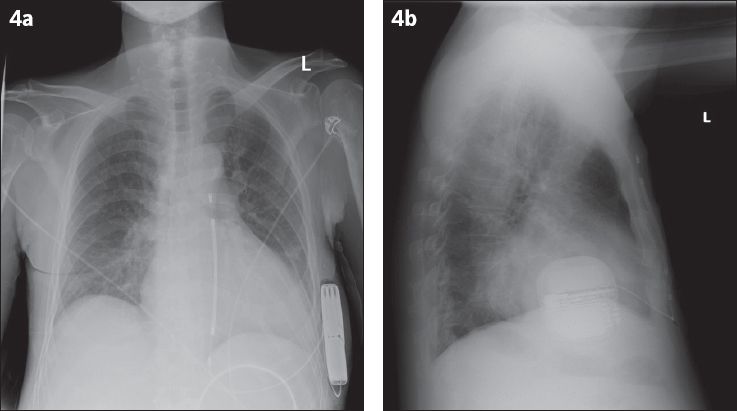
Fig. 4
(a) Posteroanterior and (b) lateral radiographs show satisfactory placement of the pulse generator of the subcutaneous implantable cardioverter defibrillator (S-ICD). The S-ICD lead travels to the inferior edge of the xiphisternum, then takes a superior course along the left parasternal margin.
Follow-up
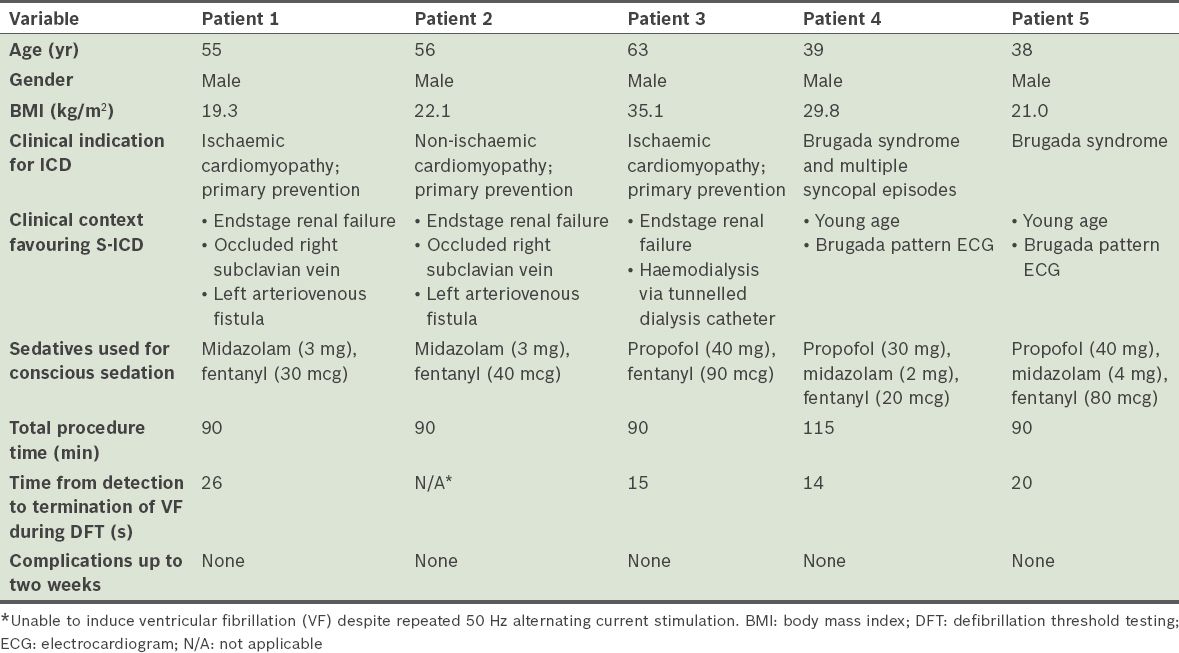
All patients underwent uncomplicated implantation of the S-ICD system. S-ICD interrogation, which was conducted immediately after S-ICD implantation, showed acceptable S-ICD lead parameters. There was no T wave oversensing in any of the patients. All patients, except Patient 2, had VF induced by 50 Hz AC stimulation and were defibrillated to sinus rhythm with a single 65 J shock by the device. The time between VF induction and delivery of the 65 J shock ranged between 14 seconds and 26 seconds. Only nonsustained VF was induced in Patient 2 despite eight attempts at 50 Hz AC stimulation (up to 10 seconds duration for each stimulation). As such, no further defibrillation testing was pursued for that patient. There were no acute complications and S-ICD interrogation within 24 hours after implantation showed stable parameters. Patients 1, 3 and 4 were discharged from the hospital a day after the procedure. Patient 2 was discharged from the hospital two days after the procedure, after heparin-free haemodialysis. Patient 5 was discharged two days after S-ICD implantation, for social reasons. All patients were reviewed within two weeks after discharge. At review, pocket and skin incisions of the patients showed normal healing and the S-ICD interrogation parameters of all patients were stable. Of note, there was no T wave oversensing in any of the patients. Patient demographics and acute implant procedural details are summarised in
Table I
Patient demographics and acute implant procedural details of the five patients who underwent subcutaneous implantable cardioverter defibrillator (S-ICD) implantation.
DISCUSSION
In the present case series, S-ICD was found to be a safe and effective alternative to transvenous ICD. S-ICD implantation is a feasible alternative for patients in whom transvenous ICD implantation is contraindicated or best avoided. Although many randomised trials have shown that conventional transvenous ICDs are effective at reducing total and arrhythmic mortality in patients at risk of SCD,(2-4) the need for a chronic indwelling vascular lead represents an important drawback. The need for at least one transvenous lead is the weakest link of the ICD system, chiefly because the lead provides a nidus for infection and must withstand repetitive mechanical stresses. It is not uncommon for younger patients to outlive the working life of a transvenous ICD lead.(8) In a series of 440 consecutive patients who underwent first implantation of an ICD, Alter et al showed that 31% experienced at least one complication within a mean follow-up duration of 46 months; one-third of the complications were lead-related.(7) Cumulative data suggests that at least 20% of transvenous leads will fail ten years after implantation.(6-9)
By eliminating the need for a transvenous lead, S-ICDs have many important advantages (
Table II
Advantages and disadvantages of subcutaneous implantable cardioverter defibrillators, as compared to transvenous implantable cardioverter defibrillators (modified from Saxon et al(20)).
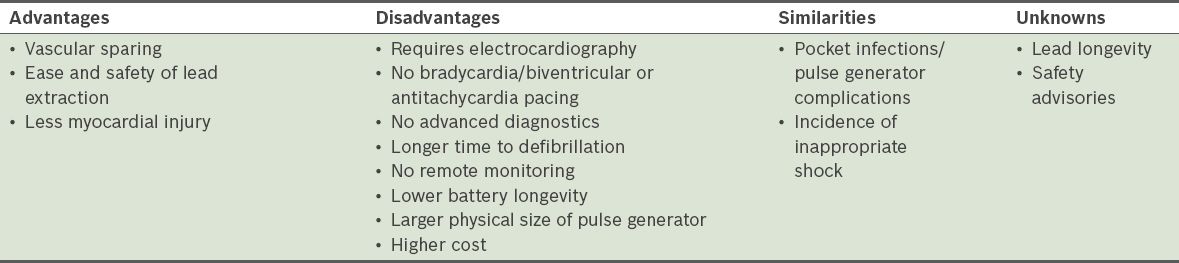
Nevertheless, the S-ICD system is not without its disadvantages. When compared to transvenous ICD systems, S-ICD systems can only provide backup pacing following a shock, as pacing from the subcutaneous position invariably causes painful muscle stimulation. Therefore, S-ICD systems cannot provide antitachycardia pacing to painlessly terminate VT. In addition, as the energy needed by the S-ICD system to defibrillate is approximately three-fold higher than that needed by transvenous ICD systems,(13,14) the pulse generator of the S-ICD system is more bulky than the current generation of transvenous ICD systems.(17,18) It measures 78 mm × 65 mm × 15 mm, with a volume of 69 cc and a mass of 145 g, which is almost twice the size of a traditional ICD. The estimated generator longevity (i.e. three full-energy capacitor charges per year) of the S-ICD system is also shorter than that of the transvenous ICD system (5 years vs. 5–8 years).
In the present case series, S-ICD implantation was safe, feasible and effective. Screening of the patients and use of the device was straightforward. The skills needed for the implantation procedure were a subset of those required for transvenous ICD implantation. We did not experience any difficulties implanting the device for any of the cases. There were initial concerns over the amount of pain the patients might experience when the subcutaneous tunnel was being made, since it is difficult to infiltrate the entire length of the tunnel with local anaesthetic. However, it was possible to achieve adequate analgesia with conscious sedation using a combination of midazolam, fentanyl and/or propofol, in conjunction with the liberal use of local anaesthetic.
In four of our five patients, VF was easily induced using 50 Hz AC stimulation and successful defibrillation was achieved with a 65 J shock. In Patient 2, we failed to induce sustained VF even though multiple attempts were made (i.e. eight times). As such, a defibrillation threshold could not be determined for Patient 2. This occurred despite the satisfactory positioning of the pulse generator and S-ICD lead. A review of the literature suggests this is an uncommon occurrence; during safety and efficacy testing of the S-ICD, failed induction of VF occurred in only 3% of the cases.(11)
In early reports on the use of S-ICD, the mean body mass index (BMI) of the patients were 28 kg/m2 and 29.7 kg/m2, respectively.(10,11) Given the relatively bulky size of the S-ICD pulse generator (compared to that of conventional transvenous ICD systems), this was a concern in the present study, as Asian patients generally have a lower BMI than Caucasian patients. The mean BMI of the five patients in the present case series was only 25.5 kg/m2. However, despite the low BMI, particularly in Patients 1 and 2, implantation of the S-ICD pulse generator in an appropriate position, without erosion of the pulse generator, was still performed successfully. Follow-up, which was conducted within two weeks after discharge, showed normal wound healing in all patients.
The S-ICD system may best serve the following three patient groups: (a) patients with prohibitively difficult vascular access, whose only other alternatives might be an epicardial ICD or transiliac ICD; (b) patients at very high risk of intracardiac device infection; for example, immunosuppressed patients or patients on haemodialysis (who frequently suffer from bacteraemia); and (c) younger patients who are expected to require one or more lead extractions/re-implantations over their lifetime. In the present study, our selection of the five patients for S-ICD implantation was guided by these considerations. Other than the three aforementioned patient groups, patients awaiting cardiac transplantation are also likely to benefit from the use of the S-ICD system.
The S-ICD system is not suitable for use in the following two patient groups: (a) patients who have a pacing requirement, including the need for cardiac resynchronisation; and (b) patients who have VT that can be easily terminated by antitachycardia pacing. In addition, up to 8% of possible candidates are found to be unsuitable for S-ICD implantation on ECG screening; hypertrophic cardiomyopathy, heavy weight, prolonged QRS duration and an R/T-wave amplitude ratio < 3 are independently associated with screen failure, which increases the risk of inappropriate therapy.(19,20) Even with these given limitations, it has been estimated that as many as 55% of patients in routine clinical practice needing an ICD are potential candidates.(21)
Clinical trials are now underway to compare S-ICD and transvenous ICD systems. The ongoing PRAETORIAN trial is a randomised, controlled, multicentre, prospective two-arm trial that aims to recruit patients with a class I or class IIa indication for ICD therapy, without indication for bradypacing or tachypacing.(22) The target sample size is 700 patients, randomised 1:1 to either S-ICD or transvenous ICD. The PRAETORIAN trial is powered for the non-inferiority of S-ICD, with respect to the composite primary endpoint of inappropriate shocks and ICD-related complications. However, real-world data from the EFFORTLESS S-ICD registry has already been published; this early report indicates that the current generation of S-ICDs already demonstrates appropriate system performance, with clinical event rates and inappropriate shock rates comparable to those reported for conventional transvenous ICD.(12)
In conclusion, the present case series demonstrated that S-ICD implantation is safe and effective for patients who meet the standard criteria for ICD therapy. S-ICD implantation is a feasible alternative, especially for patients in whom transvenous approaches are contraindicated or best avoided.


