Abstract
INTRODUCTION
This study aimed to investigate the trend and seasonality of infection due to respiratory syncytial virus (RSV) at KK Women’s and Children’s Hospital (KKH) in Singapore and to examine the risk factors for mortality among children with RSV infection requiring admission to the paediatric intensive care unit (PICU).
METHODS
A retrospective study was conducted at KKH on children with RSV infections who were admitted to the PICU between January 2004 and December 2010. The medical records of children who died from RSV infections were reviewed. Linear regression was performed to determine the risk factors for RSV mortality.
RESULTS
RSV infection was documented in 5,785 children during the study period; the infection was noted to be occurring throughout the year, with a small increase in prevalence between the months of June and August every year. Among 85 (1.5%) out of 5,785 children who were admitted to the PICU for RSV infection, 74 (1.3%) survived and 11 (0.2%) died. Multivariate logistic regression analysis showed that haemodynamically significant cardiac disease (odds ratio [OR] 12.2, 95% confidence interval [CI] 0.9–16.7, p = 0.05), immunodeficiency (OR 71.4, 95% CI 8.2–500, p < 0.001) and metabolic disease (OR 71.4, 95% CI 4.3–1,000, p = 0.003) were independent risk factors for mortality in RSV infections. Prematurity increased the risk of admission to the PICU but was not significantly associated with mortality.
CONCLUSION
Children with haemodynamically significant cardiac disease, immunodeficiency and metabolic disease were at higher risk of death after hospitalisation for RSV-related illnesses. These children should be considered for palivizumab prophylaxis.
INTRODUCTION
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections worldwide.(1) It is an important cause of a respiratory viral infection requiring paediatric intensive care unit (PICU) admission. In a national registry in France, among 467 young children hospitalised for bronchiolitis at 24 PICUs, 76% were RSV positive.(2) Hon et al reported that RSV exceeded influenza, parainfluenza and other respiratory viruses as the most common viral pathogens in the PICU of a regional hospital in Hong Kong.(3) In addition to increased morbidity, RSV infection is an important cause of childhood mortality.
Various studies have identified the risk factors for severe RSV infections that can lead to death; these include infancy, prematurity, chronic lung disease, haemodynamically significant heart disease and immunodeficiency.(4-8) Palivizumab is a humanised monoclonal antibody against the fusion protein of RSV and is effective as a prophylaxis to reduce serious RSV infections. However, it is an expensive drug, and the American Association of Pediatrics recommends that it be given only to at-risk infants during the winter months, when RSV infection is more prevalent.(9)
However, the seasonality of RSV infections in tropical climates is not well defined. Loh et al reviewed the data on the incidence of RSV infections in Singapore from 2003 to 2008.(10) They found that the incidence peaked in early June and late July. A majority of studies have shown that RSV infection is more common during the rainy season in the tropics.(11,12)
Accordingly, we aimed to describe the seasonality of RSV infection among children in Singapore from 2004 to 2010 and investigate the risk factors for mortality from RSV infection among children admitted to the PICU for it.
METHODS
This retrospective case-control cohort study investigated the seasonality of RSV infections among children admitted to a hospital in Singapore as well as the risk factors for mortality among children with RSV infections admitted to the PICU for it at the same hospital.
The microbiology records at KK Women’s and Children’s Hospital (KKH), Singapore, from 1 January 2004 to 31 December 2010 were reviewed and all children who had tested positive for RSV were included in the study. The samples were obtained from nasopharyngeal swabs and tested by immunofluorescence using the D3 Double Duet DFA Respiratory Virus immunofluorescence kit (Diagnostic Hybrids, Athens, OH, USA). The medical records of these patients were reviewed to identify patients with RSV infections who were admitted to the PICU.
Patients admitted to the PICU with RSV infections were analysed, and patients who died from the infection were compared with those who survived to identify the risk factors for mortality. Data collected included patient demographics, perinatal history, associated comorbidities, source of RSV infection, cause of death (if applicable), requirement for assisted ventilation, duration of intubation and length of stay in the PICU, and length of hospital stay.
Data was analysed using IBM SPSS Statistics version 20.0 (IBM Corp, Armonk, NY, USA). All p-values were two-tailed. Statistical significance was set at p < 0.05 for the univariate and multivariate logistic regression analyses of risk factors for mortality, duration of intubation, and durations of stay in the PICU and hospital. For univariate logistic regression analysis, categorical variables were compared using Fisher’s exact test and Pearson’s chi-square test. Categorical variables were compared with continuous variables not following normal distribution using Mann-Whitney U test. For multivariate analysis, logistic and linear regression models were used.
RESULTS
A total of 5,785 RSV-positive paediatric patients were identified during the study period, with 85 children admitted to the PICU and 11 deaths reported (
Table I
Annual frequency of RSV infections from 2004 to 2010.
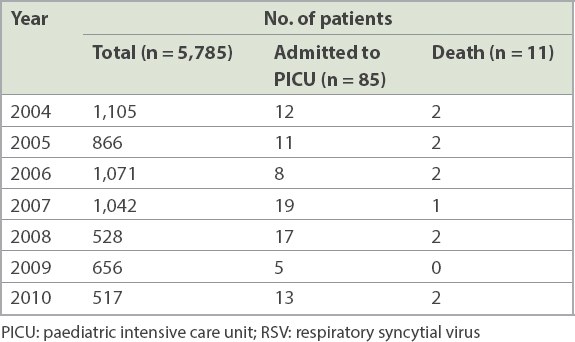
Fig. 1
Seasonality of RSV infection, as demonstrated by the monthly percentage of annual incidence from January 2004 to December 2010. RSV: respiratory syncytial virus
The clinical characteristics of children who were admitted to the PICU for RSV infection were reviewed (
Table II
Clinical characteristics of patients admitted to the PICU for RSV infection (n = 85).
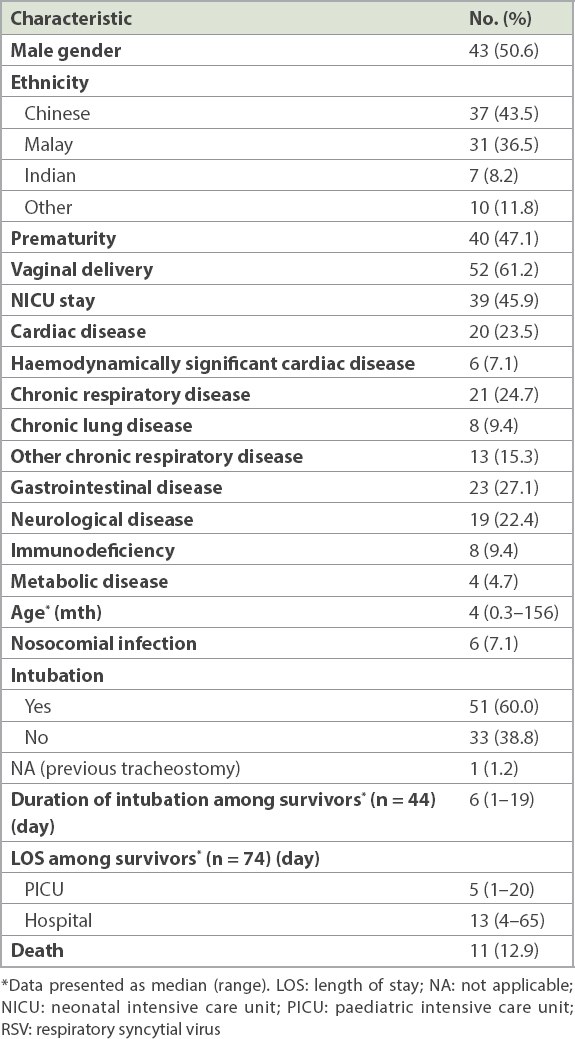
All children had concomitant underlying clinical diseases. Haemodynamically significant cardiac disease included: large ventricular septal defect, with severe mitral regurgitation, resulting in pulmonary hypertension and congestive cardiac failure; dilated cardiomyopathy; hypoplastic left ventricle, with large ventricular septal defect; large atrial septal defect, with pulmonary hypertension and congestive cardiac failure; and total anomalous pulmonary venous drainage. A premature infant who still required oxygen at Day 28 of life was defined as having chronic lung disease of prematurity. Other chronic lung disease included recurrent pneumonia, asthma, pulmonary fibrosis, tracheoesophageal fistula and restrictive lung disease. Gastrointestinal disease included gastroesophageal reflux disease, oropharyngeal dysphagia, tracheoesophageal fistula and short gut syndrome. Neurologic and neuromuscular disease included a history of intracranial haemorrhage, previous intracranial abscess, central hypotonia, arachnoid cyst, congenital hypomyelination syndrome, cerebral palsy, spinal muscular atrophy and congenital myopathy.
Among eight patients with immunodeficiency, one patient had cyclical neutropenia with recurrent pneumonia, one patient had newly diagnosed human immunodeficiency virus infection that was discovered during the admission for severe RSV infection, two patients had suspected immunodeficiency disease that had not been defined and four patients had malignancies (acute lymphocytic leukaemia, lymphoma and neuroblastoma) on chemotherapy. Among four patients with metabolic disease, three patients did not have defined disease but presented with persistent metabolic acidosis, global developmental delay and persistently raised lactate levels, and one patient had peroxisomal disorder.
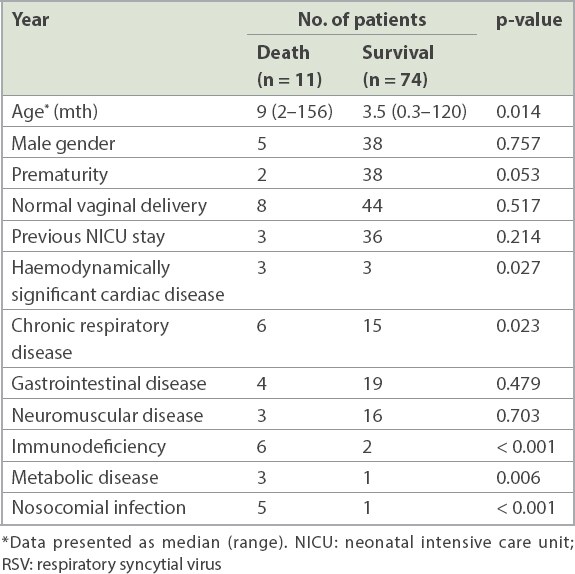
Among 85 (1.5%) out of 5,785 children who were admitted to the PICU for RSV infection, 11 (12.9%) died. Univariate logistic regression analysis of the risk factors for mortality was performed (
Table III
Univariate analysis of risk factors for mortality in patients with RSV infections.
Three out of 11 patients who died from RSV infection had other infections that contributed to mortality, such as Candida pneumonia, Staphylococcus aureus pneumonia and disseminated varicella zoster infection. Two out of these three patients had immunodeficiency. Only one out of 74 patients who survived had a concomitant pertussis infection.
44 (59.5%) out of 74 survivors required intubation. On univariate regression analysis, no risk factors were found to be associated with a longer duration of intubation. However, on multivariate regression analysis, haemodynamically significant cardiac disease (b = 13.7, 95% CI 6.3–21.2, p = 0.001), and neurologic and neuromuscular disease (b = 3.3, 95% CI 0.5–6.1, p = 0.021) were independent risk factors for a longer duration of intubation among survivors.
Prematurity was significantly associated with a longer length of stay in the PICU on univariate regression analysis. On multivariate regression analysis, neuromuscular disease (b = 3.3, 95% CI 0.7–7.0, p = 0.013) was the only independent risk factor for an increased length of stay in the PICU.
Prematurity, chronic respiratory disease, gastrointestinal disease and neuromuscular disease were risk factors for a longer hospital length of stay on univariate regression analysis. However, neuromuscular disease was the only independent risk factor on multivariate regression analysis (b = 12.6, 95% CI 7.5–17.7, p < 0.001).
DISCUSSION
RSV is the leading cause of lower respiratory tract infections worldwide. The global mortality rates for RSV infections vary depending on existing comorbidities. A recent review by Welliver et al estimated that global fatality rates among children hospitalised for RSV lower respiratory tract infections ranged from below 1% among those without underlying risk factors to as high as 37% for children with congenital heart disease.(4) Leung et al demonstrated a mortality rate of 6.8% among children in Hong Kong with severe RSV infection.(5) The annual mortality rate from RSV infection at our centre, the largest paediatric hospital in Singapore, ranged from 0% to 0.4% among all hospitalised patients with RSV infection and from 0% to 33.3% among children with severe RSV infections admitted to the PICU, especially among those with underlying comorbid diseases, in our study.
A higher proportion of Malay children compared to the ethnic mix of the Singapore population were admitted to the PICU for RSV infection in our study. This likely reflected the general attendance pattern of patients at our centre rather than ethnicity being a risk factor for PICU admission, as we do see a higher proportion of Malay children than those of other ethnicities. A similar racial distribution of more Malay patients was noted in two previous studies at our centre, where 30%–32% of our paediatric study population was ethnically Malay.(13,14)
Many studies have investigated the risk factors for severe RSV infections among children. A systematic review of 36 studies showed that chronic lung disease, congenital heart disease, prematurity, neuromuscular disease, immunosuppression, malignancies and a nosocomial source of the RSV infection were associated with mortality in children with RSV infections.(4) Another systematic review of 34 studies by Szabo et al demonstrated similar risk factors, namely chronic lung disease, congenital heart disease and prematurity.(8) Other risk factors for mortality include neuromuscular disease and chromosomal disorders.(6) Haemodynamically significant cardiac disease, immunosuppression and metabolic disorders were independent risk factors for mortality in our study. To the best of our knowledge, this is the first study to determine an association between metabolic disorders and mortality in RSV infections. Kristensen et al showed an association between inborn errors of metabolism and hospitalisation for RSV infection.(15) According to these authors, increased hospitalisation rate among children with inborn errors of metabolism was associated with them being unable to tolerate fasting, resulting in hospitalisation despite having relatively mild infections. A similar explanation can be postulated for the association between metabolic disorders and mortality in patients with RSV infections, as these children with metabolic disorders decompensate quickly during an acute infection, resulting in a more severe presentation and possible death.
Although chronic respiratory disease, neuromuscular disease and a nosocomial source of RSV infection were not independent risk factors for mortality, their association with RSV infection mortality was statistically significant on univariate logistic regression analysis. In addition, having a neuromuscular disease was the only independent risk factor for prolonged length of stay in the PICU as well as prolonged hospitalisation in our study, suggesting a more severe and protracted disease course in these patients. This was consistent with other studies.(15,16) These children have a reduced vital capacity due to muscular dysfunction or spastic scoliosis, disturbed clearance of respiratory secretions and recurrent microaspirations that contribute to increased respiratory morbidity when coupled with RSV infection.(17)
Palivizumab is a humanised murine monoclonal antibody that provides passive immunity against RSV infections.(18) According to the American Academy of Pediatrics, palivizumab is recommended during the peak RSV season for infants at risk of severe RSV infection in the first year of life. These include premature infants born before 29 weeks of gestational age, those with chronic lung disease and those with haemodynamically significant heart disease. It can also be considered for children with pulmonary abnormalities or neuromuscular diseases that impair the ability to clear secretions from the upper airways in their first year of life and for children aged below 24 months who are severely immunocompromised.(9) The findings of our study support these guidelines, as haemodynamically significant heart disease and immunodeficiency were independently associated with mortality in patients with RSV infection, and chronic respiratory disease was associated with mortality on univariate logistic regression analysis. Currently, at our centre, palivizumab is recommended for neonates with gestational age below 29 weeks, children aged below two years requiring treatment for chronic lung disease within the preceding six months and children aged below one year having haemodynamically significant congenital heart disease.
In temperate climates in the northern hemisphere, the peak season for RSV infections is in winter, between November and March.(19) In the tropics, it is more difficult to identify a well-defined season for RSV. A local study by Loh et al looked at correlations between clinical illness, respiratory virus infections and climate factors in a tropical paediatric population from 2003 to 2008. These authors reported that RSV infections tend to peak in early June and late July in Singapore.(10) This concurred with our observation that RSV infection peaked between June and August. However, an older local study by Chew et al that looked at data from 1990 to 1994 reported that RSV infections peaked in May and June.(20) This discrepancy could be attributable to changes in the local climate, as it is postulated that, in tropical countries, the incidence of RSV infection correlated positively with the monthly total rainfall and mean average temperatures.(10,11,20-23)
This study has some limitations. Our sample size was small, which limited its statistical power to identify other independent risk factors that may have been related to mortality in RSV infections. Further, this was a retrospective review of the clinical charts and medical records of patients, a methodology that is limited by the accuracy of the documented information available.
Mortality from RSV infection was generally low in healthy children with no underlying comorbid diseases. However, children with haemodynamically significant cardiac disease, immunodeficiency and metabolic disease were at a higher risk of death if they were hospitalised for an RSV-related illness. Palivizumab prophylaxis is recommended for children at risk of severe RSV infection and should be given during the RSV season. The challenge in tropical countries is that there is no well-defined season for these infections, as RSV is present throughout the year, with a slight increase observed during the months from June to August in Singapore. These children will need to receive palivizumab throughout the year, especially in the first year of life, as long as they are deemed to still be at risk. A more cost-effective route of prevention would involve the development of a vaccine that is effective against RSV.


