Abstract
Colonoscopy with endoscopic resection of detected colonic adenomas interrupts the adenoma-carcinoma sequence and reduces the incidence of colorectal cancer and cancer-related mortality. In the past decade, there have been significant developments in instruments and techniques for endoscopic polypectomy. Guidelines have been formulated by various professional bodies in Europe, Japan and the United States, but some of the recommendations differ between the various bodies. An expert professional workgroup under the auspices of the Academy of Medicine, Singapore, was set up to provide guidance on the endoscopic management of colonic polyps in Singapore. A total of 23 recommendations addressed the following issues: accurate description and diagnostic evaluation of detected polyps; techniques to reduce the risk of post-polypectomy bleeding and delayed perforation; the role of specific endoscopic resection techniques; the histopathological criteria for defining endoscopic cure; and the role of surveillance colonoscopy following curative resection.
INTRODUCTION
Colorectal cancer (CRC) is the most common cancer in Singapore. Diverse genetic and epigenetic changes underlie the initiation and progression of the adenoma-carcinoma sequence in colorectal carcinogenesis.(1) Colonoscopy with endoscopic resection (ER) of detected colonic adenomas interrupts the adenoma-carcinoma sequence and has been shown to reduce the incidence of CRC(2) and CRC-related mortality.(3) The European Society of Gastrointestinal Endoscopy (ESGE) published a set of guidelines on colorectal polypectomy and endoscopic mucosal resection (EMR) in 2017(4) and endoscopic submucosal dissection (ESD) in 2015.(5) Apart from cold snare polypectomy (CSP),(6) EMR and ESD, there have been further advances in ER technique, such as endoscopic full-thickness resection (EFTR), using a scope-mounted full-thickness resection device (FTRD).(7) These techniques are now part of routine clinical practice in Singapore.(8,9) There is currently no clinical guidance on colonoscopy ER techniques in Singapore. It is recognised that there cannot be uniformity in the choice of techniques, with technical variations necessitated by expertise and specific patient characteristics. However, it is important to provide evidence-based guidance to optimise treatment outcomes by increasing the success rates of curative ER and minimising procedure-related complications.
The Specialty Board of the Chapter of Gastroenterologists of College of Physicians, Academy of Medicine, Singapore (AMS), initiated the formation of a workgroup in January 2019 to address the issue of ER during colonoscopy under the auspices of the AMS. This workgroup was formed with representatives from the Chapter of Gastroenterologists of the College of Physicians, Chapter of General Surgeons of the College of Surgeons, Gastroenterological Society of Singapore and Society of Colorectal Surgeons Singapore. An expert gastrointestinal pathologist was also invited to join the workgroup (Appendix). The aim of the workgroup was to review the current evidence for ER during colonoscopy and draft a set of recommendations to harmonise and improve the quality of practice in Singapore. ER is but one part of the overall management strategy to reduce CRC incidence and mortality. Screening of at-risk individuals through faecal immunochemical test or colonoscopy,(10) good-quality bowel preparation during colonoscopy,(11) high-quality colonoscopy(12) and appropriate surveillance strategies are as important.(13) These recommendations do not define a standard of care but are written in the spirit of further heightening endoscopic practice standards. In clinical practice, variations may be needed and would be acceptable, based on patient characteristics and endoscopist expertise.
METHODS
A set of key clinical questions (CQs) was first formulated. These CQs addressed the following areas: (a) the role of endoscopic diagnosis of colonic polyp prior to resection; (b) role of endoscopic techniques to reduce the risk of post-resection complications; (c) roles of specific resection techniques; and (d) criteria for defining endoscopic cure. Workgroup members were assigned to address each CQ. Within each CQ, a draft set of evidence-based recommendations was formulated. The 2017 ESGE guidelines were reviewed, and a PubMed search of relevant published literature up to April 2020 was performed. The quality of evidence and strength of recommendation of each statement were assessed according to GRADE (Grading of Recommendations, Assessment, Development and Evaluations;
Box 1
Grading of Recommendations Assessment, Development and Evaluation (GRADE): Quality of evidence

Box 2
Recommendations for endoscopic management of colonic polyps:

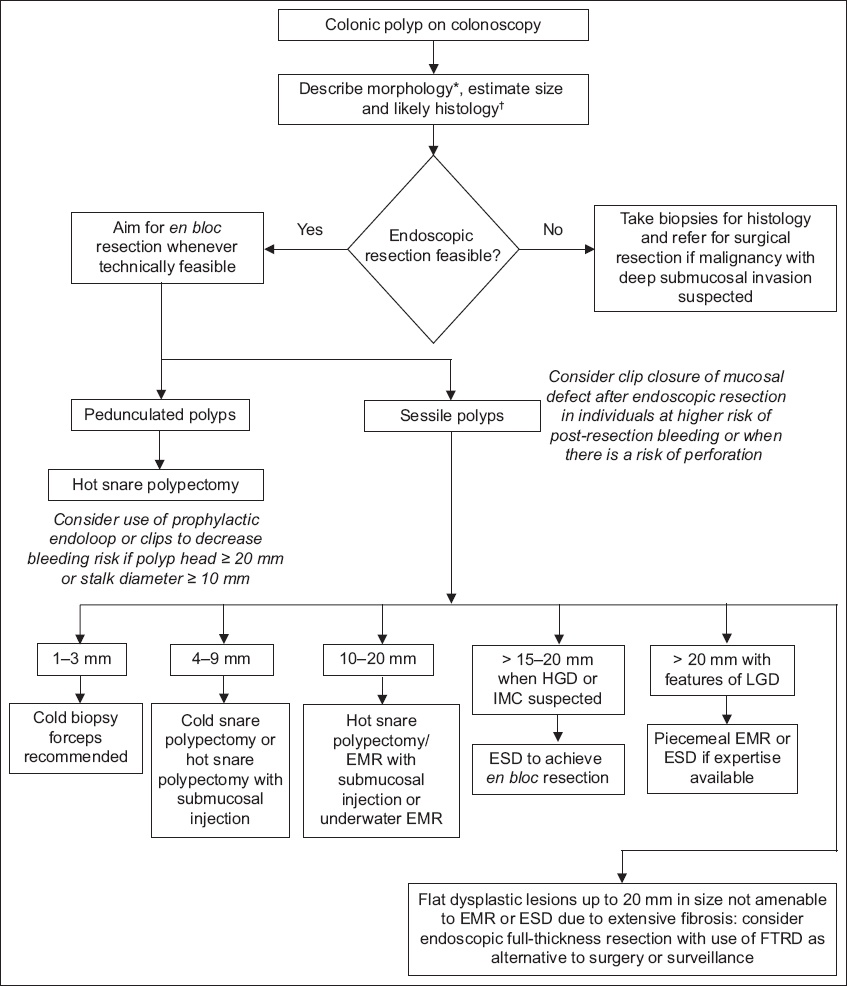
Fig. 1
Flowchart shows the endoscopic management of colonic polyps. *Paris classification. †NICE (no magnification) or JNET (with magnification) classifications. EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; HGD: high-grade dysplasia; IMC: intramucosal cancer; LGD: low-grade dysplasia; FTRD: full-thickness resection device; JNET: Japan NBI Expert Team; NICE: NBI International Colorectal Endoscopic
RECOMMENDATIONS
(A) Endoscopic evaluation of colonic polyps prior to resection
An accurate diagnostic evaluation is important to guide the decision for further therapeutic intervention. A precise terminology to describe findings is crucial. Adjuncts to standard white light endoscopy provide additional diagnostic information that aids polyp characterisation.
The gross morphology of a polyp facilitates estimation of the depth of invasion, which may influence the choice of resection techniques. A prediction of deep invasion is further confirmed when the lesion fails to lift after injection of submucosal saline (the non-lifting sign). Documentation of morphology also provides a common vocabulary to facilitate communication between endoscopists when patients are referred for ER. The Paris endoscopic classification of superficial neoplastic lesions, updated in 2005, is internationally accepted.(15) Superficial lesions are classified as protruding, non-protruding and non-excavated, and excavated. Protruding lesions are described as pedunculated (0–Ip) or sessile (0-Is). Non-protruding and non-excavated lesions are described as slightly elevated (0–IIa; < 2.5 mm in height, or the width of biopsy forceps, as opposed to sessile lesions that are > 2.5 mm), completely flat (0–IIb) and slightly depressed (0–IIc; < 2.5 mm in depth). Excavated lesions are ulcerated lesions (0–III). Combinations of morphologies may exist for a single lesion such as elevated and depressed (0–IIa + IIc) or excavated and depressed (0–III + IIc) lesion. The availability of such a morphological description is very important for histopathologists in their interpretation of resected malignant polyps. The Haggitt classification is used for pedunculated lesions,(16) whereas for sessile or flat lesions, the measurement of vertical depth of extension into the submucosa is crucial(17) when assessing whether ER is curative. This will be further discussed in Section D. Gross morphological features such as convergence of folds, spontaneous bleeding and surface ulceration are indications of deeper invasion and should be documented if present. A documented description makes a difference to the decision to perform the polypectomy there and then, the subsequent plan for endoscopic resection, the approach should a redo polypectomy be required and surgical decision-making if surgery is ultimately required.
Image-enhanced endoscopy (IEE) has moved from the niche practice of expert endoscopists to mainstream clinical practice. IEE enhances mucosal surface and microvascular patterns to facilitate diagnostic evaluation. IEE techniques are classified as dye-based (chromoendoscopy [CE]) or equipment-based.(18) Equipment-based IEE has greatly simplified IEE, as the endoscopist only needs to press a button on the scope handle without having to administer a dye, and it is also less messy than dye-spray. Moreover, endoscopes manufactured by the major endoscopy companies are now equipped with IEE technology.
Equipment-based IEE is further categorised into optical IEE (such as narrow-band imaging [NBI; Olympus, Tokyo, Japan], blue laser and blue light imaging [BLI; Fujifilm, Tokyo, Japan], and optical enhancement [OE; Pentax, Tokyo, Japan]) and electronic IEE based on software processing (such as i-scan [Pentax] and Fuji Intelligent Colour Enhancement [FICE; Fujifilm]). Optical equipment-based IEE is preferred over electronic IEE owing to its better image resolution. Dye-based CE enhances the pit pattern of the colonic polyp, whereas equipment-based optical IEE, such as NBI, BLI and OE, use the narrow-bandwidth blue and green wavelengths to improve the visualisation of mucosal pit patterns and microvessels of the colonic polyp. These mucosal surface changes have been correlated with the histology and depth of submucosal invasion in malignant polyps.(19-23) Although these details can be examined with and without the use of magnification, magnification is important for clear visualisation of the shape and calibre of microvessels. A commonly used system to predict polyp histology is the NBI International Colorectal Endoscopic (NICE) classification, which is based on three parameters, namely colour, vessels and surface pattern of the polyp.(19,20) It does not require image magnification. NICE 1 has been correlated with hyperplastic polyp or sessile serrated polyp (SSP); NICE 2 with adenomatous polyps with low-grade dysplasia (LGD), high-grade dysplasia (HGD) or intramucosal carcinoma (IMC); and NICE 3 with malignant polyps with deep submucosal surface invasion. The Japan NBI Expert Team (JNET) classification system requires the use of magnifying endoscopy to examine the vessel and surface patterns. It consists of four categories of microvessel and surface pit patterns (Types 1, 2A, 2B and 3). Types 1, 2A, 2B and 3 are correlated with the histopathological findings of hyperplastic polyp/SSP, LGD, HGD/IMC/shallow submucosal invasive cancer and deep submucosal invasive cancer, respectively.(21,24-26) In essence, with the use of optical magnification, one can further stratify a lesion that is classified as NICE 2 into 2A and 2B, as there are implications for the choice of ER technique, as well as whether en bloc resection is required (HGD, IMC) or piecemeal resection would suffice (LGD).
The use of optical magnification with magnifying or dual-focus endoscopy can enable the endoscopist to visualise microsurface and microvascular patterns more clearly, providing further information for classification of polyps. A systematic review and meta-analysis compared the accuracy of NBI, magnifying chromoendoscopy (MCE), and gross morphological features (GMF) seen with conventional view for the optical diagnosis of T1 CRC and deep submucosal invasion. Altogether, 33 studies with 31,568 polyps were included. For the optical diagnosis of T1 CRC, both NBI (four studies; pooled estimate [PE] 0.85, 95% confidence interval [CI] 0.75–0.91) and MCE (five studies; PE 0.90, 95% CI 0.83–0.94) yielded higher sensitivity than GMF (three studies; range 0.21–0.46). Similarly, for the optical diagnosis of deep invasion, both NBI (13 studies; PE 0.77, 95% CI 0.68–0.84) and MCE (17 studies; PE 0.81, 95% 0.75–0.87) yielded higher sensitivity than GMF (six studies; range 0.18–0.88). No significant preference for either NBI or MCE was found.(27) ER should not be attempted in NICE 3 or JNET 3 lesions. In JNET 2B lesions consistent with HGD or IMC, one should attempt to achieve en bloc resection to ensure complete ER, rather than perform piecemeal ER. It must be acknowledged that these classification systems are not perfect and that ultimately, a histological assessment is still required to determine the success of endoscopic treatment.
Polyps that are classified as NICE 3 or JNET 3 have a high risk of deep submucosal invasion, which is associated with increased risk of nodal metastasis. ER will not be curative even if technically successful.(16,17,28) Attempting ER may result in perforation.(29) Referral for surgical resection is indicated.
(B) Endoscopic techniques to reduce the risk of post-resection complications
Colonoscopy with ER is a very safe procedure, with a low risk of procedure-related complications. The key complications that may arise are immediate and delayed bleeding, and perforation. The magnitude of the risk depends on patient-specific characteristics and the choice of ER techniques, which is, in turn, guided by the type of lesion and the technical limitations of each technique. Most complications, when recognised early, are successfully treated endoscopically without the need for rescue surgery. Based on factors such as clinical comorbidities, polyp morphology and appearance of the post-resection mucosal defect, specific preventive measures may be taken for risk mitigation.
Data regarding the use of haemostatic clips to prevent post-polypectomy bleeding (PPB) is mixed. A case-control study showed that prophylactic clip placement did not reduce the occurrence of PPB.(30) This data corroborated the findings of a previous randomised controlled trial (RCT).(31) However, some studies involving resection of large polyps showed reduction in the risk of PPB. In a study by Watabe et al involving 6,617 polypectomies, PPB occurred at a rate of 0.57%. Based on this, the number of clips needed to be placed prophylactically to prevent a single episode of post-polypectomy bleeding was 175.(32) This is neither practical nor cost-effective. Haemostatic clips can be used to treat immediate active bleeding but need not be placed routinely after standard polypectomy. A recent systematic review evaluated 4,311 patients and 7,783 polyps. There was no significant difference in delayed PPB rates in patients who received routine prophylactic clipping compared to those who did not (odds ratio [OR] 0.8; 95% CI 0.36–1.77; p = 0.56). There was also no significant difference in the delayed PPB rates of polyps < 20 mm compared with polyps ≥ 20 mm.(33) A more recent RCT published in 2019 again demonstrated that routine prophylactic clip placement for polyps > 10 mm did not reduce the rate of delayed bleeding.(34) A multicentre RCT showed that bleeding after endoscopic removal of large non-pedunculated colon polyps (≥ 20 mm) is reduced by the prophylactic placement of haemostatic clips to close the mucosal defect only for large polyps located in the proximal colon.(35)
This statement should be read together with Statement 13, which recommends hot snare resection for pedunculated polyps. It is consistent with the ESGE recommendation.(4) Studies have shown the efficacy of endoloop in preventing PPB after resection of large pedunculated colon polyps.(36,37) In an RCT of 195 patients who had pedunculated colorectal polyps with heads ≥ 10 mm and stalks ≥ 5 mm in diameter, polyps were randomised to receive either clips or endoloop. Both devices were applied to the base of the stalk before conventional snare polypectomy. Bleeding occurred after five polypectomies in the clip group and after six polypectomies in the endoloop group (5.1% vs. 5.7%; p = 0.847). These results suggest that the application of a clip may be as effective and safe as an endoloop in the prevention of PPB in large pedunculated colonic polyps.(38) A single endoloop is expected to completely strangulate the vessels within the polyp stalk, whereas depending on the diameter of the polyp stalk and the size of the clips, more than one clip may need to be deployed.
The risk of PPB is significantly increased in patients on antiplatelet or anticoagulation therapy, as well as in patients with end-stage kidney disease. The Endoscopic Resection Group of the Spanish Society of Endoscopy (GSEED-RE) model(39) and the Australian Colonic Endoscopic Resection (ACER) model(40) have been proposed to predict delayed bleeding after EMR of large superficial colorectal lesions. A multicentre cohort study assessed the discrimination and calibration of the GSEED-RE and ACER models in a validation study and proposed a newer predictive model. In this study, PPB occurred in 45 of 1,034 EMR of lesions > 20 mm (4.5%) and was associated with proximal location (OR 2.84, 95% CI 1.31–6.16), antiplatelet agents (OR 2.51, 95% CI 0.99–6.34) or anticoagulants (OR 4.54, 95% CI 2.14–9.63), difficulty of EMR (OR 3.23, 95% CI 1.41–7.40) and comorbidity (OR 2.11, 95% CI 0.99–4.47). These variables were then used to generate a new model, GSEED-RE2, which achieved higher area under the curve values (0.69–0.73, 95% CI 0.59–0.80).(41) Prophylactic clip placement after ER of large polyps has been shown to be cost-effective for such patients at higher risk of post-resection bleeding.(42) Such a strategy should apply to individuals who are expected to have a higher risk of post-resection bleeding (lesion size ≥ 20 mm at proximal colon location,(35) presence of major comorbidities, use of antiplatelet therapy). Careful evaluation of the post-resection mucosal defect is important. If there is endoscopic evidence to suggest deep mural injury that may result in delayed perforation, or if there is a high risk of perforation (‘target sign’), mechanical prophylactic closure of the mucosal defect is indicated.(29,43)
(C) Specific endoscopic resection techniques
There has been development in ER techniques such that a spectrum of options is now available. A brief definition will be provided here, as some terms have been used interchangeably and can be a source of confusion.
En bloc resection refers to resection of the entire polyp in a single piece, whereas piecemeal resection refers to resection of the polyp in multiple fragments. In theory, a diminutive polyp may be completely removed by biopsy. Normally, this is done without cautery and is known as cold biopsy. Hot biopsy refers to avulsion of the polyp using electrocautery; these are specially designed biopsy forceps that allow electrocautery. Standard snare polypectomy refers to the resection of a polyp by placing a snare at its stalk or base and using electrocautery. It is also known as hot snare polypectomy (HSP). CSP refers to resecting a polyp without cautery. It is performed without submucosal injection. Dedicated cold snares specifically designed for CSP are now commercially available.
EMR is an extension of standard SP techniques. It is used to resect non-polypoid lesions. EMR differs from HSP in that the plane of resection is the middle to deep submucosal layer, as opposed to the mucosal or the most superficial submucosal level for HSP. Hence, EMR is able to provide adequate tissue to assess the depth of invasion even if the lesion is mildly elevated or flat, rather than pedunculated or sessile. Submucosal injection to expand the submucosa is traditionally used to separate the mucosa layer from the muscularis propria layer and to avoid deep thermal injury during resection. EMR requires the use of a dedicated stiff snare that can be firmly anchored on the colonic mucosa to capture both the mucosa and submucosa tissue. To ensure that there is no remnant lesion at the resection margin during EMR, it is crucial that a rim of normal mucosa around the lesion is also captured and resected. In the oesophagus and stomach, as the mucosa and submucosa layers are thicker than those in the colon, the use of snare resection for EMR may not be able to capture the layers adequately to obtain a clear margin, and hence, EMR techniques that create pseudo-polyps by suction prior to resection, such as cap-assisted and ligation-assisted EMR techniques, are used; these are, however, not used for colonic EMR owing to the thin colonic wall and risk of perforation. Underwater EMR is a newer alternative to resect non-pedunculated colorectal lesions without submucosal injection. It is based on the observation that mucosal lesions float away from the muscle layer once immersed in water.
ESD uses dedicated endoscopic electrocautery knives to incise the mucosal layer and then dissect the submucosal layer beneath the non-polypoid lesion. The mucosa layer is first separated from the muscularis propria layer by submucosal injection during ESD, similar to EMR. Unlike EMR, where the snare limits en bloc resection to lesions < 20 mm, the use of electrosurgical knives in ESD enables en bloc resection of lesions beyond a diameter of 20 mm. As ESD can be technically challenging for the non-experts, the concept of hybrid ESD has been advocated. With hybrid ESD, there is circumferential mucosal incision around the non-polypoid lesion, followed by limited submucosa dissection to create sufficient excavation to allow a snare to capture the polyp easily for resection; this will also minimise the risk of deep thermal injury and perforation from colonic wall tenting owing to capture of excessive tissue. EFTR refers to complete resection of the colonic wall layer from the mucosa to the muscularis propria.
Piecemeal vs. en bloc endoscopic resection
En bloc resection provides tissue for histopathological assessment in a single piece. Piecemeal resection results in multiple tissue fragments, and the completeness and adequacy of resection cannot be objectively determined and commented upon by the histopathologist. IMC resected piecemeal would invariably be offered surgery even if ER is curative, since histological features that support curative resection cannot be assessed. The implications for patients may be significant, as surgery has both short- and long-term complications. In addition, there is a risk of residual disease and local recurrence with piecemeal resection. For pedunculated lesions, en bloc resection is not an issue if the snare is large enough to loop over the head of the polyp and ensnare the stalk. The challenge lies in non-pedunculated lesions that are flat, mildly elevated or sessile. In such circumstances, lesions < 15 mm, and even up to 20 mm, can be captured by a stiff snare and resected safely. For lesions < 10 mm, en bloc resection is preferable over piecemeal biopsy to allow for more accurate histologic assessment for completeness of ER.
A systematic review and meta-analysis that addressed the issue of recurrence of non-pedunculated lesions after EMR included a total of 33 studies in the analysis. Recurrence risk was significantly higher after piecemeal resection (20%, 95% CI 16%–25%) than after en bloc resection (3%, 95% CI 2%–5%; p < 0.0001). In multivariable analysis, only piecemeal resection was associated with recurrence.(44) There is a risk of deep mural injury, and even perforation, when en bloc resection is attempted by snare resection or EMR for non-pedunculated lesions > 20 mm owing to the effect of tenting. A recent study analysed data from a prospective tertiary referral multicentre of lateral spreading tumours (LSTs) 20–25 mm in size that were referred for EMR over a ten-year period. The risk of major deep mural injury was significantly higher in the en bloc EMR group compared to the piecemeal EMR group (3.5% vs. 1.0%, p = 0.05). Residual or recurrent adenoma at first surveillance was significantly lower in the en bloc EMR group than in the piecemeal EMR group (2.0% vs. 5.7%, p = 0.04), but this difference was negated at subsequent surveillance. The importance of en bloc resection in assessing the possibility of curative endoscopic resection was highlighted by the following observation. Among LST with endoscopic suspicion of malignancy in the en bloc resection group, nine had evidence of invasive cancer at histopathology, of which 5 (56%) were assessed as low risk for submucosal invasion and considered endoscopically cured. Of the eight high-risk LSTs in the piecemeal EMR group, 4 (50%) had evidence of invasive cancer at histopathology but could not be considered for endoscopic cure owing to piecemeal resection.(45) To achieve en bloc resection for larger non-pedunculated lesion, ESD would be required, and depending on the expected histology, expertise and risk-benefit considerations, one would have to either consider an attempt at ESD to achieve en bloc resection or accept piecemeal resection using EMR. Another systematic review with meta-analysis compared ESD with EMR for non-pedunculated lesions > 20 mm. The en bloc resection rate was 89.9% for ESD patients vs. 34.9% for EMR patients (relative risk [RR] 1.93, p < 0.001). The R0 resection rate was 79.6% for ESD patients vs. 36.2% for EMR patients (RR 2.01, p < 0.001).(46)
The adequacy of ER in this case centres on two key determinants: (a) the complete excision of the areas of HGD and invasion by IMC; and (b) the absence of histopathological features that predispose the patient to a higher risk of lymph node metastasis (LNM). Both of these determinants will require en bloc or at least near en bloc resection.(45,46) The histopathological criteria required to confirm curative ER(28) will be further elaborated on in Section D of the paper.
Complete histological assessment is essential to confirm curative endoscopic treatment in HGD and IMC, and this requires that the lesion be resected in its entirety in a single piece. This may not be so crucial in LGD, and local recurrent disease when detected during surveillance can be treated by ER. The caveat is that a small proportion of lesions may be upstaged after ER and histological assessment.(9) The efficacy of adjuvant thermal ablation of the EMR mucosal defect margin in reducing polyp recurrence was examined in a multicentre randomised trial from Australia. After complete resection by EMR, lesions were randomly assigned to thermal ablation of the post-EMR mucosal defect margin (n = 210) or no additional treatment (controls, n = 206). Surveillance colonoscopy was performed after 5–6 months. A significantly lower proportion of patients who received thermal ablation of the post-EMR mucosal defect margin had evidence of recurrence than the controls (5.2% vs. 21%, p < 0.001; RR 0.25, 95% CI 0.13–0.48).(47)
Hot biopsy and cold biopsy
Hot biopsy forceps (HBF) employ thermal ablation with a coagulation current through an electrosurgical unit to achieve resection of polyps. When the polyp is grasped in the forceps, electrocautery is applied to destroy the polyp base, while polyp tissue is preserved inside the forceps as a histological specimen. It is an easy-to-use technique with minimal obstruction of the endoscopic view during application, high specimen retrieval rate and short procedure time. This may be of particular advantage in the case of polyps where visibility or access is difficult around a corner or fold. HBF has been viewed as an effective method of enhancing complete resection rate and inducing simultaneous haemostasis because of the additional effect of electrocautery ablating the surrounding tissue. However, the use of HBF may potentially confound histological diagnosis of the retrieved specimen owing to thermal damage. In two studies with a combined total of 162 diminutive polyps removed using HBF, more than 90% of resected specimens showed some cautery damage or artefact. However, 80%–83% of them remained of sufficient overall diagnostic quality to support histological diagnosis.(48,49) HBF also carries some risk of adverse events such as delayed bleeding or hypercoagulation syndrome. A retrospective survey carried out in 1987 reported 6 (0.05%) perforations, 47 (0.4%) episodes of bleeding and one death out of 12,367 HBF procedures.(50) However, more recent studies conducted in 1995–1997 with a combined total of 2,432 polyps removed by HBF found significant bleeding in 0%–0.4% of cases, with no perforations.(51,52) Similarly, an RCT conducted in 2017 comparing HBF and CSP reported no perforations and no statistically significant difference in occurrence rates of immediate bleeding between the two techniques (9% vs. 8%, p > 0.05).(53) In a retrospective review of 323,585 polypectomies, higher odds of adverse events were reported among endoscopists with low annual polypectomy volume.(54) The ability of HBF to completely ablate any dysplastic tissue has been questioned. Studies show rates of remnant polyp tissue ranging between 11% and 21% when HBF was used for diminutive polyps.(55-58) Considering the available evidence on its complete resection rate, safety and histological quality, we recommend caution in the use of hot biopsy forceps.
Similar to HBF, cold biopsy forceps (CBF) are effective and easy to use, with a high specimen retrieval rate and short procedure time. Due to the absence of electrocautery, the risk of adverse event is limited to immediate post-biopsy bleeding, which will stop spontaneously in most instances or can be easily controlled by application of clips. Blood loss at the biopsy site, however, might hinder visualisation, contributing to an increased risk of incomplete polypectomy and consequent risk of interval CRC. Varying completing resection rates from CBF of diminutive polyps, ranging from 29% to 83%, have been reported in previous studies.(55,58-60) A 2016 meta-analysis of five RCTs with a total of 668 patients and 721 polyps concluded that incomplete histological eradication of polyp tissue was significantly lower with CSP or jumbo biopsy forceps (JBF) techniques than with CBF (RR 0.40; 95% CI 0.29–0.62).(61) However, an RCT published in 2016 by Park et al showed no statistically significant difference between the complete resection rates of CBF and CSP (91% vs. 93%) if NBI was applied after CBF to ensure no remnant polyp was visible endoscopically.(62) Typical biopsy forceps have a diameter of 4–5 mm, which can plausibly allow one-bite complete resection of a 1–3 mm polyp, but may prove challenging for larger polyps. Jung et al reported a higher complete resection rate by CBF of 96% in 1–3 mm polyps compared to 89% in polyps > 3 mm; however, this difference was not statistically significant.(63) Two studies comparing the effectiveness of CBF to CSP and JFB, respectively, in removing diminutive polyps found no difference, specifically in polyps < 4 mm.(59,64) However, lower resection rates for CBF were observed in polyps ≥ 4 mm in both studies (70% vs, 94% for CSP; 83% vs, 100% for JBF). Considering the available evidence on its complete resection rate compared to other polypectomy techniques, we recommend that CBFs be used for polyps 1–3 mm in size, while for polyps > 3 mm in size, CSP or JBF is preferred.
Cold and hot snare resection
The rationale for HSP in pedunculated polyps is the observed presence of thick vessels in the polyp stalk.(65,66) HSP allows coagulation of these vessels in the polyp stalk. This must be read in context of Statement 6, where it is recommended that prophylactic measures such as endoloop or clip application to prevent bleeding in pedunculated polyps with polyp head size ≥ 20 mm or stalk diameter ≥ 10 mm be used.(36-38,67)
CSP was first introduced in 1992.(68) In recent years, it has started to gain greater popularity owing to the availability of dedicated snares that are thinner and sharper and can resect effectively without cautery, unlike traditional snares.(69) Unlike HSP, submucosal saline injection is not required as a buffer against thermal injury, thus speeding up the process of CSP. Immediate bleeding tends to occur, but it stops spontaneously as only superficial capillaries are involved. The CRESCENT RCT demonstrated that for sessile polyps measuring 4–9 mm, the complete resection rate between CSP and HSP was similar (98.2% vs, 97.4%, non-inferiority p < 0.0001). Bleeding requiring endoscopic haemostasis occurred only with HSP (0.5%).(70) These results were further supported by a meta-analysis where the risk ratio of incomplete resection of polyps ≤ 10 mm using CSP compared with HSP was similar (1.36, 95% CI 0.92–2.01).(71)
Before the recent popularity of CSP, HSP was the standard approach. The CRESCENT study showed similarly low rates (< 5%) of incomplete resection for both CSP and HSP.(70) Studies have also shown similarly low rates of complications.(70,71)
HSP is the current standard approach for resection of sessile polyps between 10 and 20 mm. In most instances, owing to concern about deep thermal injury, submucosal injection prior to HSP is performed. For a flat, non-polypoid lesion, saline injection is needed to lift up the diseased mucosa so that it can be captured by a snare. Such an approach was also strongly recommended by ESGE for polyps measuring 10–19 mm in size.(4) There is emerging data concerning the use of CSP for polyps > 10 mm. However, for polyps of this size, CSP cannot achieve en bloc resection, and piecemeal resection is required. In a study conducted at a tertiary centre, piecemeal CSP without submucosal injection for sessile serrated polyps without endoscopic evidence of dysplasia 10–35 mm (median 15 mm) in size was carried out on 41 polyps, and there was no evidence of recurrence at a median of six months’ follow-up. There was no evidence of perforation, significant intraprocedural bleeding, delayed bleeding or post-polypectomy syndrome.(72) An RCT randomised 52 patients with rectal or rectosigmoid polyps ≤ 10 mm in diameter to HSP or CSP and assessed the resection width and the depth achieved. Muscularis mucosa was obtained similarly with HSP and CSP (96%, 95% CI 82%–99% vs. 92%, 95% CI 75%–98%; p = 0.603). However, submucosal tissue was obtained significantly more frequently with HSP than with CSP (81%, 95% CI 63%–92% vs. 24%, 95% CI 11%–43%; p < 0.001). This highlights the limitation of CSP in obtaining sufficient resection depth for adequate assessment in the presence of dysplasia.(73) A previous study retrospectively analysed 1,006 colorectal polyps 2–14 mm in size resected with CSP. With respect to the difference between lesions ≥ 10 mm and those < 10 mm, the rates of cancer and positive/unclear margins were significantly higher (5.0% vs. 0.9%, p < 0.001; 40.6% vs. 27.7%, p = 0.007) in polyps ≥ 10 mm. There was also loss of muscularis mucosa in 27.8% of lesions.(74)
Underwater EMR is a technique of hot snare resection that was first introduced in 2012 and gained popularity in recent years. It can be considered an option to hot snare resection after submucosal injection. The non-pedunculated lesion is immersed in water, allowing the mucosa to float away from the muscularis propria layer such that ER can be achieved without a need for submucosal injection. It may also allow a larger resection since the mucosa layer is collapsed and more tissue can be captured by the snare.(75) A systematic review and meta-analysis of published non-randomised data up to May 2018 pooled data from ten studies with 433 patients. The complete resection rate was 96.36% (95% CI 91.77–98.44), with an en bloc resection rate of 57.07% (95% CI 43.20–69.91). The recurrence rate was 8.82% (95% CI 5.78–13.25) during a mean endoscopy surveillance period of 7.7 (range 4–15) months. The post-procedural bleeding rate was 2.85% (95% CI 1.64–4.90). Bleeding during the procedure was always mild and was considered part of the procedure in all series. The overall adverse event rate was 3.31% (95% CI 1.97–5.52). No cases of perforation were reported.(76) A Japanese RCT published in 2019 compared underwater EMR with conventional EMR for intermediate-size sessile colorectal polyps measuring 10–20 mm. The proportion of R0 resections was 69% (95% CI 59–77) in the underwater EMR group vs. 50% (95% CI 40–60) in the conventional EMR group (p = 0.011). The proportion of en bloc resections was 89% (95% CI 81–94) in the underwater group vs.75% (95% CI 65–83) in the conventional EMR group (p = 0.007). There was no significant difference in the median procedure time (165 seconds vs. 175 seconds) or proportion of patients with adverse events.(77)
Endoscopic mucosal resection and endoscopic submucosal dissection
When there is a suspicion of HGD or IMC, it is crucial to achieve en bloc resection with sufficient normal mucosal margin and depth of submucosa to allow precise histopathological assessment. When lesions are resected piecemeal, there may be cautery effects across the areas of HGD and IMC, and it is also difficult to correctly orientate the specimens such that the histopathologist will not be able to ascertain definitively whether the lesion has been completely resected. In addition, adverse prognostic factors associated with risk of metastatic disease, such as foci of lymphovascular invasion or tumour budding, may be obscured. EMR is not able to resect lesions > 20 mm en bloc, and piecemeal resection is needed. Indeed, even for smaller lesions 15–20 mm in size, ESD rather than EMR may still be required if there is difficulty ensnaring the lesion with adequate clear margins or when there is fibrosis. This may require either a complete ESD procedure or a hybrid ESD procedure. ESD provides the possibility of en bloc resection. A meta-analysis has clearly shown that the R0 resection rate of ESD is significantly higher than that of EMR, and the risk of recurrence is significantly lower for sessile lesions > 20 mm. A total of 11 case control or cohort studies with 4,678 patients were included in the analysis, of which 1,517 (32.4%) had an ESD and 3,161 (67.6%) had an EMR. There was no RCT. Indications for EMR and ESD were lesions > 20 mm. The mean polyp size in the ESD series was 33.7 mm vs. 27.4 mm in the EMR series (p < 0.001). The en bloc resection rate was reported in eight studies (89.9% in ESD group and 34.9% in EMR group; RR 1.93, 95% CI 1.46–2.54, p < 0.001). The R0 resection rate was reported in four studies (79.6% in ESD group and 36.2% in EM group; RR 2.01, 95% CI 1.76–2.29, p < 0.001). The recurrence risk was reported in ten studies (rate of 0.7% in ESD group and 12.7% in EMR group; RR 0.06, 95% CI 0.03–0.11, p < 0.001).(46) Data from a Japanese series demonstrated the long-term effectiveness of this technique, with three- and five-year overall/disease-specific survival rates of 97.1%/100% and 95.3%/100%, respectively.(78) There are no comparative studies with surgery, but ESD is less invasive and organ preserving. A systematic review published in 2012 showed an adequate safety profile for colorectal ESD, with a negligible (1%) risk of post-ESD complication-related surgery compared with the high efficacy of this procedure. Almost all ESD complications were managed endoscopically. In that study, a cumulative risk of 6% for bleeding and perforation was reduced to a 1% risk for complication-related surgery because of the efficacy of endoscopic treatment of ESD-related complications.(79) Surgery should be considered for lesions involving more than half the circumference, when severe fibrosis prevents adequate submucosal expansion by submucosal injection for ESD or when the expertise for ESD is lacking.
As mentioned in Section A, careful endoscopic evaluation prior to attempting ER using advanced techniques such as EMR and ESD is crucial to ensure that only lesions with LGD are subject to piecemeal resection. Classification systems such as NICE and JNET help to predict histology. However, one must remember the caveat that there are no perfect systems. With large lesions, part of the mucosal surface may not be sometimes clearly seen, or more severely dysplastic regions could reside in a deeper layer of the polyp. Lesions have been upstaged from initial biopsy findings after ER.(9,80,81) The ACE (Australian Colonic Endoscopic) resection study group conducted a prospective, multicentre, observational study of 479 patients referred for EMR of sessile colorectal polyps ≥ 20 mm. In this cohort, risk factors for submucosal invasion were as follows: Paris classification 0–IIa+c morphology, non-granular surface and Kudo pit pattern Type V. The most commonly observed lesion (0–IIa granular) had a low rate of submucosal invasion (1.4%). EMR was effective at completely removing the polyp in a single session in 89.2% of patients. Independent predictors of recurrence after EMR were lesion size > 40 mm and use of argon plasma coagulation.(82) The same group later published the recurrence rate of a prospective cohort of 1,000 patients with sessile or laterally spreading colonic lesions ≥ 20 mm in size treated by EMR. Surveillance colonoscopy was performed at four (early) and 16 (late) months after EMR. Early recurrent/residual adenoma was present in 16% of patients and late recurrent/residual adenoma was uncommon (4%). Risk factors for recurrent adenoma were lesion size > 40 mm, use of argon plasma coagulation to ablate adenoma and intraprocedural bleeding. The recurrent adenoma was usually diminutive and was successfully managed endoscopically in 93.1% of cases.(83)
There is a concern of undetected HGD and IMC in such lesions, and lesions have been upstaged after ER.(9,80,81) This may pose a management dilemma after piecemeal resection, with patients then referred for salvage surgery even if curative ER had been achieved, because it is not possible to confirm R0 resection. En bloc resection is associated with higher rates of R0 resection and lower recurrence. Although EMR can theoretically achieve en bloc resection for lesions up to 20 mm, it does not consistently achieve en bloc resection for lesions that are 15–20 mm in size.(77) Although the meta-analysis by Arezzo et al reported a rate of perforation of 4.9% for the ESD group and 0.9% for the EMR group (RR 3.19, p < 0.001), most cases were managed conservatively with endoscopic clip closure without the need for surgery. The bleeding rate for ESD was similar to that for EMR (1.9% vs. 2.9%, p = 0.070).(46) In addition, very low rates of complications – 1.7% for bleeding and 1.9% for perforation – have been reported when ESD is performed by experts.(84) It is, therefore, an issue of managing the risk-benefit equation. Patients need to be informed of such considerations during the consent process.
Endoscopic full-thickness resection
Conventional resection techniques and other advanced techniques such as ESD and EMR require adequate submucosal lifting with injection solution prior to safe resection. This may be impossible or technically challenging in the setting of severe fibrosis from a desmoplastic reaction, scarring from an incomplete polypectomy, and tattoo injection performed to mark the location of the lesion. FTRD (Ovesco Endoscopy, Tübingen, Germany) is an over-the scope system that allows single-step EFTR after placement of a modified over-the-scope clip (OTSC). The endoscope with the mounted FTRD is introduced into the colon and the target lesion is identified. The grasping forceps (Ovesco Endoscopy) are advanced through the working channel of the endoscope to grasp the lesion and pull it into the cap mounted at the tip of the scope, in order to capture a double full-thickness layer of the colonic wall. With the lateral margins of the lesion pulled into the cap, the OTSC is deployed. The pseudopolyp created by the OTSC is then resected using the pre-loaded snare, while the OTSC secures integrity of the colonic wall.(85) A recent prospective, multicentre study demonstrated that the overall technical success and R0 resection rates for EFTR were 89.5% and 76.9%, respectively.(7) The majority of lesions reported in the study were difficult adenomas with a benign final histology. Difficult adenomas were defined as either non-lifting or located in difficult anatomical locations such as the diverticula and at the appendiceal orifice. This group of polyps had a high technical success rate of 92.1% and an R0 resection rate of 77.7%. The rates of technical success and R0 resection for subepithelial tumours in the study were 95.5% and 87.0%, respectively. Most recent retrospective studies studying EFTR for various indications comprised mainly non-lifting adenomas or adenomas in difficult anatomical locations. The technical success rate ranged from 82.3% to 100%, while the R0 resection rate varied between 82.3% to 87.9%.(86-88) Procedure-related adverse event rate in the largest multicentre prospective study to date was reported to be 9.9%. These adverse events consisted mainly of perforation (3.3%), bleeding (2.2%) and post-polypectomy syndrome (1.7%).(7)
This recommendation is due to the inability to obtain sufficient margin to ensure R0 resection in this setting when using FTRD to perform EFTR. From a technical perspective, given the size of the distal cap, it will be difficult to ensure that the entire lesion is pulled in with clear margin for a polypoid lesion, unlike in a situation with non-polypoid lesions. In an early experimental animal study where FTRD was used in live pig colons, the average diameter of the tissue resected with FTRD in the three groups of pigs was 3.1 cm, 3.6 cm and 5.4 cm, respectively.(89) However, the pig colonic wall is thinner than the human colonic wall. The technical success and R0 resection rates have been shown to dramatically decrease with increasing lesion size. In the largest prospective study by Schmidt et al,(7) the technical success and R0 resection rates of EFTR were 100% and 87.5%, respectively, for lesions ≤ 9 mm. However, for lesions > 20 mm, the technical success and R0 resection rates were 79.0% and 58.1%, respectively. This is a concern when FTRD is used for EFTR of polyps > 20 mm. A large, multicentre retrospective study evaluating EFTR in T1 CRC reported an overall technical success and R0 resection rates of 92% and 72%, respectively. The median lesion size in the study was 20 mm.(90) The R0 resection rate for non-lifting malignant polyps was significantly lower than that for incompletely resected malignant polyps (60.9% vs. 87.5%). Moreover, despite the apparent R0 resection achieved, two patients who belonged to the group with previous incomplete resection and who were initially classified as low risk based on final histological analysis had residual or recurrent disease. The follow-up data in this study was for only three months, and a longer follow-up period could well reveal more recurrences, as T1 CRC can recur much later.(91) The frequency of cancer in lesions > 20 mm is also higher compared to smaller lesions.(92) EFTR is not recommended in the context of suspected deep submucosal invasion because it will be non-curative, as explained in Section D.
(D) Criteria for defining endoscopic cure
ER is a local treatment. To be considered curative, the lesion should not have a significant risk of nodal or distant metastasis. Surgical-histopathological correlation and long-term outcome data have demonstrated that well-defined histopathological criteria can be established and used to define cure for lesions treated endoscopically.
A malignant polyp is defined as one with invasion through the muscularis mucosa. Cure may be achieved through ER. However, 8%–16% of malignant polyps may show LNM, which has a significant impact on cure and survival.(93-96) The five-year survival for a malignant polyp without LNM (Stage I) was > 95%, while the presence of LNM (Stage III) reduced overall five-year survival to 68.4%–87.6%.(97) Surgical resection with lymph node dissection is necessary for malignant polyps with suspected LNM, to ensure curative resection and improve survival. The predictive value of histological features for LNM is, thus, of clinical interest. There have been five meta-analyses published since 2013 on histopathological predictive factors for LNM in malignant polyps. The common histological features identified as significantly associated with LNM risk across multiple studies were: (a) depth of submucosal invasion > 1,000 mm; (b) lymphatic invasion; (c) vascular invasion; (d) poor differentiation; and (e) tumour budding.(98-102) In addition to these criteria, the European Society of Medical Oncology and American Society for Gastrointestinal Endoscopy guidelines(103,104) included involved margins of resection and Level 4 invasion based on the Haggitt system in pedunculated polyps as other unfavourable histological findings.(16) Patients who do not meet these criteria for cure should be referred for surgical resection with nodal dissection, unless there are severe medical comorbidities precluding surgery and/or the patients are willing to accept the risk of occult metastatic disease and subsequent recurrence without surgery, which may not be an absolute certainty.(105)
Colonoscopy after surgical resection of CRC aims to detect synchronous CRC or adenomas in the context of obstructed colon or inadequate bowel preparation during preoperative colonoscopy, and to detect metachronous or missed lesions during surveillance.(13) In early CRC treated by ER, colonoscopy is repeated to detect local residual disease from inadequate ER. Endoscopic follow-up of the polypectomy site to ascertain the absence of residual disease is necessary to ensure optimal outcomes in the context of piecemeal resection, or when the clearance of resection margins cannot be determined by histopathological assessment. A retrospective analysis of 140 low-risk and 480 high-risk T1 cancers from the Netherlands Cancer Registry revealed that the rates of incomplete resection were 0.7% in the former group and 4.4% in the latter.(106) The joint consensus guidelines from the British Society of Gastroenterology, Association of Coloproctology of Great Britain and Ireland, and Public Health England strongly recommended a site check to be performed 2–6 months after piecemeal EMR or ESD of large non-pedunculated colorectal polyps (LNPCPs; at least 20 mm in size) to look for residual disease, followed by a further site check at 18 months from the date of the original resection to detect late recurrence, at low evidence.(107) Additionally, these guidelines strongly recommended that as recurrence rates with pathologically en bloc R0 resection after EMR and ESD of LNPCPs or early polyp cancers are low, no site checks are required, and the patient should undergo post-polypectomy surveillance after an interval of three years.(107) ESGE guidelines strongly recommended endoscopic surveillance 3–6 months after successful index treatment, and in the absence of recurrence, a follow-up colonoscopy was recommended one year later. After piecemeal resection or with the presence of positive lateral margins without indication for surgery, surveillance colonoscopy with biopsies at three months was recommended (low-quality evidence).(5) The United States multi-society task force on CRC(13) recommended surveillance colonoscopy one year after CRC resection, as studies have shown that the one-year examination was high yield and cost effective.(108)
We recommend assessment of the resection site within 3–6 months after curative ER of malignant polyps to ensure the absence of residual disease in the context of piecemeal resection, or if complete resection cannot be definitively established by histopathological assessment. If there is R0 resection from en bloc resection, the first follow-up colonoscopy should be at one year. In the absence of disease recurrence at one year, colonoscopy is recommended three years later and then at five-yearly intervals until the benefit of continued surveillance is outweighed by diminished life expectancy.(13,107)
CONCLUSION
These recommendations serve as a guide to the practising endoscopist in Singapore for endoscopic management of colonic polyps. Precise diagnostic evaluation and description, strategies to mitigate the risk of complications, the role of specific resection techniques and the definition of endoscopic cure are addressed. They are based on available published evidence to date and expert opinion when data is lacking. These recommendations do not define a standard of care but are written in the spirit of further heightening endoscopic practice standards. In clinical practice, variations may be needed and would be acceptable, based on patient characteristics and endoscopist expertise.
SUPPLEMENTARY MATERIAL
The Appendix is available online at https://doi.org/10.11622/smedj.2020108.


