INTRODUCTION
Sacubitril/valsartan is a first-in-class angiotensin receptor-neprilysin inhibitor (ARNI). It inhibits neprilysin via sacubitrilat, an active metabolite of sacubitril, and the effects of angiotensin II, via valsartan. The PARADIGM-HF study demonstrated the superior efficacy of sacubitril/valsartan (up-titrated to a target dose 200 mg twice daily) compared with enalapril (target dose 10 mg twice daily) in reducing mortality and morbidity, with comparable safety outcomes, in patients with heart failure (HF) with reduced ejection fraction (HFrEF).(1) Sacubitril/valsartan has since been approved for treatment of HFrEF in many countries, having received Class IB recommendation in the American and European HF management guidelines.(2,3)
Asian HF patients possess unique clinical characteristics and often have worse outcomes than HF patients from the West.(4,5) In the ADHERE-AP (Acute Decompensated Heart Failure Registry-Asia Pacific) registry of 10,171 patients hospitalised with HF in eight Asia-Pacific countries, Asian HF patients were younger than those in Western populations (median age 67–70 years vs. 70–75 years in United States and Europe).(4) Regulatory approval of HF drugs in Asian countries has largely been based on results of clinical trials in Western populations. The application of and adherence to guideline-directed medical therapy (GDMT) in Asia may differ from the Western experience. For instance, the use of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) was similar between ADHERE-AP and ADHERE, but there was lower uptake of beta-blockers and higher use of mineralocorticoid receptor antagonists in ADHERE-AP than in ADHERE.(4)
PARADIGM-HF enrolled 1,509 subjects (18% of total number of subjects) from Asian countries. There were no treatment interaction effects on the efficacy benefits by race or by geographical region.(1) Prior small studies suggested that genetic variation in Asians may result in different pharmacodynamic and pharmacokinetic profiles of drugs acting on the renin-angiotensin-aldosterone system.(6-9)
In this observational study, we report the initial experience of ARNI use in a Singapore cohort, including patient characteristics, drug efficacy and safety, clinical outcomes, and biomarker response.
METHODS
This was a retrospective analysis of a single-centre open-label observational registry of consecutive HF patients initiated on ARNI at the National Heart Centre Singapore. The study was approved by our institutional review board.
Male or female patients aged ≥ 21 years who were diagnosed during hospital admission or at ambulatory specialist HF clinics to have chronic HFrEF (ejection fraction ≤ 40%) with New York Heart Association (NYHA) Class II or more symptoms were enrolled. A washout period of ≥ 36 hours was mandated in subjects who were on ACEI, and ARNI dosages were up-titrated based on tolerability and blood pressure levels at physicians’ discretion. Subjects who were included in the analysis had to: (a) consume at least one dose of ARNI and (b) have N-terminal-pro hormone B-type natriuretic peptide (NT-proBNP), creatinine and potassium levels taken at baseline and three months. Patients who could not be initiated on ARNI due to any of the following reasons were excluded: symptomatic hypotension, systolic blood pressure < 100 mmHg, estimated glomerular filtration rate < 30 mL/min/1.73 m2 body surface area, serum potassium level above the upper limit of normal (> 5.1 mmol/L), history of angio-oedema, known allergy or intolerance to ARBs, or prior exposure to ARNI.
Primary efficacy outcomes were changes in NYHA class and NT-proBNP levels at three months compared to baseline. Primary safety outcomes were changes in serum creatinine and potassium levels at three months compared to baseline. Exploratory efficacy outcomes of interest included cardiovascular death, death from any cause, hospitalisation for HF, and left ventricular assist device implantation or heart transplant. Exploratory safety outcomes of interest included treatment-emergent symptomatic hypotension, worsening renal function, hyperkalaemia and angio-oedema. Events were ascertained from reviewing case records linked to the hospital’s electronic medical records. Information on deaths was obtained from the Registry of Births and Deaths, Singapore.
Only descriptive statistics were reported. Categorical variables were presented as numbers and percentages. Continuous variables were presented as median with interquartile range (IQR) or as mean ± standard deviation (SD) as appropriate. Paired t-tests were used to compare baseline and three-month parameters. STATA version 13 software (StataCorp LP, College Station, TX, USA) was used to perform all analyses.
RESULTS
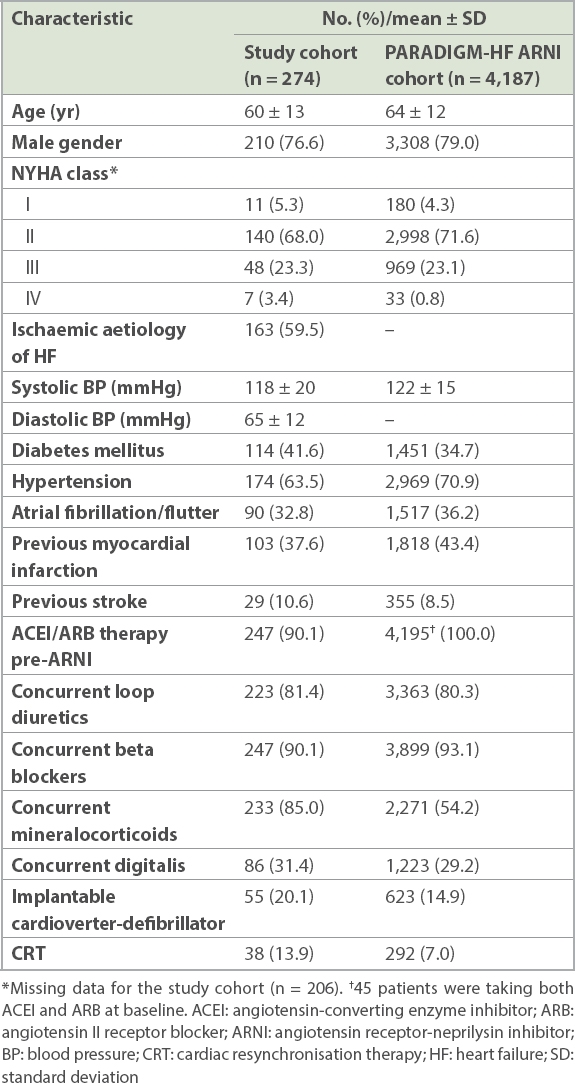
From 1 November 2015 through 31 December 2016, 274 patients were initiated on ARNI at the institution: 65 (23.7%) during hospital admission and 209 (76.3%) at the ambulatory HF clinic. Their mean age was 60 ± 13 years, and 210 (76.6%) were male. Most patients were receiving the recommended pharmacologic therapy for chronic HF at baseline. 90.1% of the cohort were on either an ACEI or ARB prior to starting the ARNI (
Table I
Baseline characteristics of our study cohort versus subjects randomised to ARNI in the PARADIGM-HF study.
ARNI was discontinued in 41 (15.0%) patients, and the dose was reduced in 12 (4.4%) patients. Mean doses at initiation and at last assessment were 120 ± 49 mg and 161 ± 97 mg daily, respectively. Among subjects who stopped ARNI, the mean duration on treatment was 2.2 ± 2.0 months.
There were significant reductions in mean NYHA class (2.2 ± 0.6 vs. 1.8 ± 0.6; p < 0.001) and median NT-proBNP levels (3,041 [IQR 1,570–7,095] pg/mL vs. 1,284 [IQR 574–3,506] pg/mL, p < 0.001) at three months compared to baseline. Death from cardiovascular causes occurred in 6 (2.2%) patients. Death from any cause occurred in 9 (3.3%) patients. 47 (17.2%) patients were hospitalised for HF, while 3 (1.1%) patients received left ventricular assist devices or heart transplants. There were no significant differences between baseline and three-month serum creatinine (107 ± 37 mmol/L vs. 112 ± 43 mmol/L; p = 0.2) and potassium (4.3 ± 0.4 mEq/L vs. 4.3 ± 0.5 mEq/L; p = 0.7) levels.
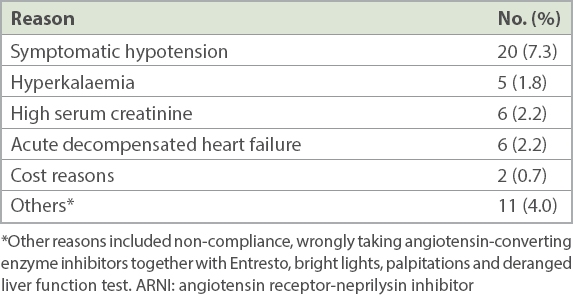
Treatment-emergent adverse events included cough (n = 6, 2.2%), hyperkalaemia (n = 5, 1.8%), renal impairment (n = 6, 2.2%) and hypotension (n = 20, 7.3%). No patient had angio-oedema, airway compromise or required mechanical airway protection. 8 (2.9%) patients were clinically assessed to have developed renal impairment and hyperkalaemia that was sufficient to warrant withdrawal of ARNI (
Table II
Reasons for stopping or reducing ARNI dosage (n = 274).
DISCUSSION
In our study, the composite outcome of death from cardiovascular causes and hospitalisation for HF was 19.2% over approximately five months. This was compared to 21.8% over a median follow-up duration of 27 months in the PARADIGM-HF ARNI treatment group. Baseline characteristics of our subjects were similar to those of subjects who were randomised to ARNI in PARADIGM-HF (
ARNI led to an improvement in NYHA class and NT-proBNP levels at three months compared to baseline and is generally well-tolerated. Decline in renal function occurred in only 2.2% of patients (similar to PARADIGM-HF), with no significant changes in serum creatinine and potassium levels at three months. Symptomatic hypotension (6.2% vs. 16.7% in PARADIGM-HF) was the main reason for ARNI discontinuation. No incident airway compromise was recorded.
Despite shorter follow-up, more subjects discontinued ARNI in our cohort than in PARADIGM-HF (15.0% vs. 10.7%). It is likely that there was less motivation and support for drug continuation in a non-trial setting. Although ARNI is largely paid out of pocket in Singapore, few discontinued it for cost reasons alone in our initial experience (
In summary, Asian HF patients possess unique clinical characteristics and often have worse outcomes than HF patients from the West.(4,5) Our real-world experience corroborates the efficacy outcomes and safety results of PARADIGM-HF. This registry data contributes to an increasing body of evidence that underscores the need for dedicated pharmacodynamic and dose titration studies specific to Asian populations, without which differences in appropriate drug dosages in this population will remain unknown.
This study was not without limitations. Our sample size was small, with a short follow-up period. As with observational studies, it was prone to selection bias, missing data and measurement error. Surrogate measures using NT-proBNP and NYHA class status do not equate to survival, efficacy and improved quality-of-life measures. There was also no control group for comparison. However, as guidelines have recommended the use of ARNI(5) and it is now widely available with salutatory symptomatic benefits, it would not be ethical to deny patients this treatment as a control arm in a study. Nevertheless, given the paucity of data in Asian-centric populations, our results are noteworthy to demonstrate tolerability and favourable effects on surrogate outcomes, at lower ARNI doses than in the PARADIGM-HF trial population.
In conclusion, ARNI use at a lower achieved dose than in PARADIGM-HF improved NYHA class and NT-proBNP levels in our multi-ethnic Singapore HFrEF population, and was well-tolerated. Our initial experience adds to real-world evidence on ARNI use and lends support to the inclusion of ARNI as GDMT in Asian patients.


