Abstract
We herein report the use of endoscopic n-butyl-2-cyanoacrylate injections to obliterate a gastric varix, which led to cyanoacrylate embolisation in the splenic and portal veins in a single patient. Cyanoacrylate embolisation is a known but uncommonly reported complication of endoscopic sclerotherapy. This case report illustrates the successful management of this complication (i.e. cyanoacrylate embolisation in the splenic and portal veins) with anticoagulation and analyses the presentation and management of other cases of cyanoacrylate embolisation reported in the literature.
INTRODUCTION
Gastric varices account for 10%–15% of all variceal bleeding and are an uncommon but important cause of upper gastrointestinal bleeding.(1) The use of n-butyl-2-cyanoacrylate injections to obliterate varices has been shown to be effective and is currently widely accepted as the therapy of choice for bleeding gastric varices.(2) However, a rare but known complication of the therapy is portal embolisation of cyanoacrylate. We herein report a case of splenic and portal vein emboli after successful haemostasis of a bleeding gastric varix, and discuss the possible consequences of this complication. A review of reported cases and the use of anticoagulation in the management of our patient is also presented.
CASE REPORT
A 73-year-old man with no history of liver disease presented to our unit with a one-week history of melaena without haematemesis. His blood pressure was 99/40 mmHg and he had a pulse rate of 84 beats per minute. There was conjunctiva pallor and non-icteric sclerae, but no splenomegaly. Digital rectal examination confirmed the presence of melaena. Laboratory tests revealed the following: haemoglobin 8.2 (normal range [NR] 14.0–18.0) g/dL; platelet count 205,000 (NR 140,000–440,000)/µL; and normal liver function tests and coagulation screen. Serologic markers were negative for hepatitis B and C. Upper endoscopy showed a nodular gastric fundal varix with an overlying clot; the varix started to bleed profusely after the clot was dislodged. Haemostasis was secured with 2.4 mL of a 1:1.4 mixture of n-butyl-2-cyanoacrylate and lipiodol, administered in two injections.
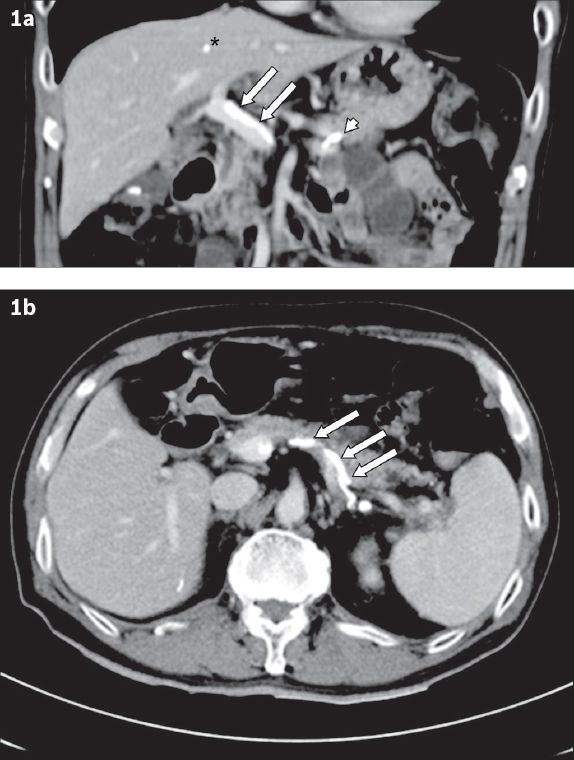
After endoscopic haemostasis, computed tomography (CT) of the abdomen was performed to investigate the cause of his possible portal hypertension. CT showed the absence of liver cirrhosis, but the presence of sclerosant in the treated gastric varix. It also showed evidence of embolisation of cyanoacrylate in the main portal vein, splenic vein and liver (
Fig. 1
Abdominal CT images one day after sclerotherapy show cyanoacrylate embolism in (a) the main portal vein (arrows), splenic vein (arrowhead) and liver (asterisk); and (b) the splenic vein (arrows).
Doppler ultrasonographic assessment of the portal system was performed to assess portal venous flow. It showed no colour flow at the distal portal vein, but spectral Doppler demonstrated a biphasic waveform, indicating partial blockage of the main portal vein. However, the right and left portal veins were patent and had normal phasic spectral waveforms. The patient had an isolated and transient rise of serum aspartate transaminase level to a peak of 42 (NR 15–33) IU/L. After a multidisciplinary discussion, the patient was deemed to be able to benefit from cautious anticoagulation to maintain portal venous flow, in view of the thrombogenic cyanoacrylate embolus within the main portal vein. He was started on subcutaneous enoxaparin (Clexane) 40 mg once a day for five days after sclerotherapy. No further episodes of gastrointestinal bleeding was noted; he was discharged and maintained on anticoagulation therapy for three months.
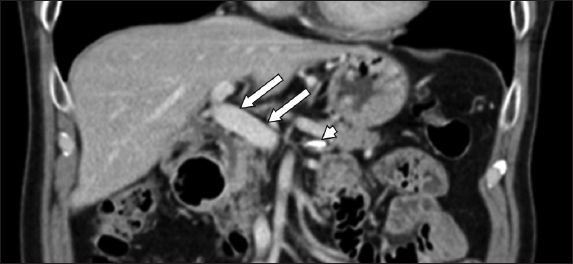
Doppler ultrasonography of the hepatic vasculature one month later showed a patent main portal vein with normal phasic waveforms. Subsequent CT imaging in six months showed no evidence of sclerosant within the main portal vein. There was minimal sclerosant in the splenic vein, which remains patent, and significantly less sclerosant in the liver parenchyma. No hepatic atrophy was noted (
Fig. 2
Abdominal CT six months after completion of anticoagulation therapy shows patent main portal vein with no sclerosant within (arrows) and minimal sclerosant within the splenic vein (arrowhead).
DISCUSSION
Bleeding from gastric varices is a life-threatening condition with a one-year mortality rate of 52%.(3) Cyanoacrylate is an adhesive that polymerises on contact with tissue moisture to form a firm, stable mass within the varix. In the largest series (635 patients over 10 years) reported by Cheng et al,(4) endoscopic injection of cyanoacrylate had a 95% success rate of achieving initial haemostasis and has emerged in many centres worldwide as the initial treatment of choice for acute bleeding gastric varices. Complications of this method are rare and include the following: recurrence of bleed due to early extrusion of the glue cast (4.4%); sepsis (1.3%); distant emboli (pulmonary, cerebral, splenic; 0.7%); gastric ulcer formation (0.1%); major gastric variceal bleeding (0.1%); and mesenteric haematoma associated with haemoperitoneum and bacterial peritonitis (0.1%). The reported complication-related mortality rate was 0.53%.(4)
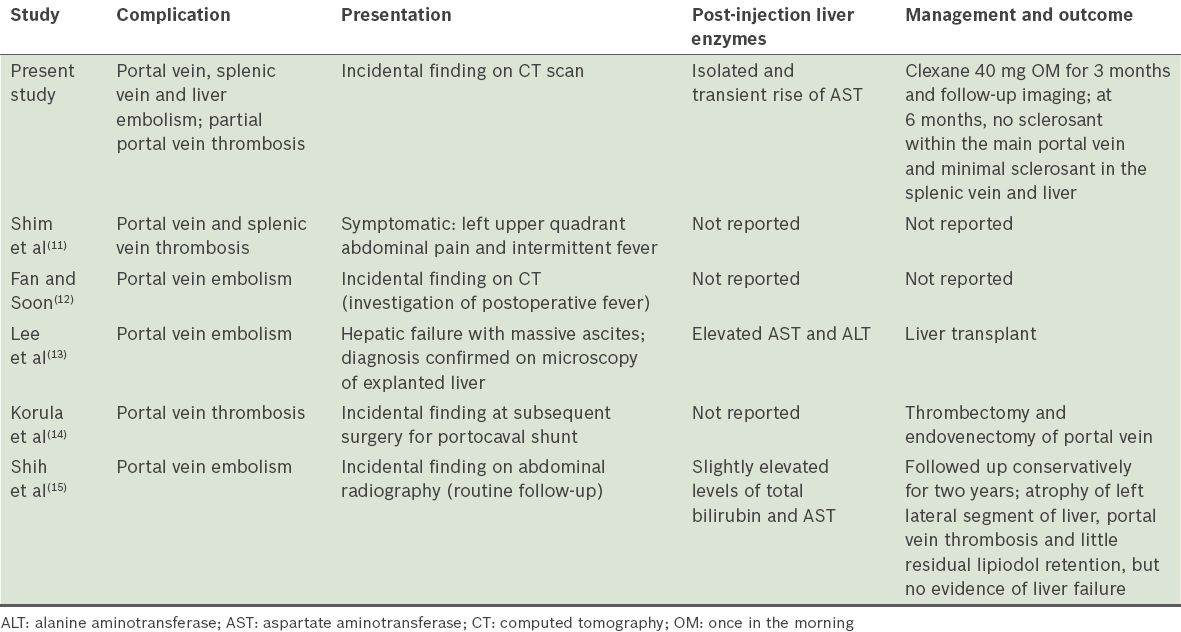
Sclerosant embolisation may occur in the systemic splenic, mesenteric or portovenous circulation. Within the systemic circulation, pulmonary, cerebral and coronary embolisations have been described and such emboli may be life threatening.(5) In contrast, a systematic review of the literature provided only five other reports of portovenous embolisations, which showed varied courses. A summary of their presentations, laboratory findings, and management and outcome are presented in
Table I
Presentation, management and outcome of portovenous cyanoacrylate embolism case reports in the literature.
Cyanoacrylate emboli within the portovenous system, if large enough, may acutely obliterate the portal vein. More commonly, it gives rise to acute or chronic portal vein thrombosis due to the thrombogenic property of cyanoacrylate. Acute portal vein thrombosis (APVT) usually presents with colicky abdominal or lumbar pain of sudden onset or progressing over a few days. Partial thrombosis may be associated with few symptoms and is commonly discovered only during radiologic examination for other reasons.(6) The potential consequences of APVT, regardless of aetiology, include intestinal infarction and portal hypertension leading to gastrointestinal bleeding. The aim of APVT treatment is the prevention of complete thrombosis and the recanalisation of the obstructed veins. Treatment modalities that have been reported to be successful include anticoagulation and thrombolysis given into the systemic venous circulation, the superior mesenteric artery or the portal vein via the transjugular or transhepatic route. In a study by Condat et al, 90% of patients achieved some degree of recanalisation and major complications were reported in less than 5%.(6) In contrast, the efficacy of thrombolysis is lower and mortality increased, but should be considered if initial anticoagulant therapy fails.(7) The American Association for the Study of Liver Diseases practice guidelines recommend giving anticoagulation therapy for at least three months, starting with low-molecular-weight heparin and shifting to oral anticoagulation.(8) Turnes et al showed that anticoagulation initiated within 30 days of the onset of symptoms was associated with recanalisation of the portal vein in about 40% of patients, compared to patients who were not anticoagulated (none achieved portal vein recanalisation).(9) Should the obstructed veins fail to recanalise, chronic portal vein thrombosis (CPVT) may develop. The clinical manifestations of CPVT include gastrointestinal bleeding from ruptured oesophageal or gastric varices, a degree of subclinical encephalopathy, and splenomegaly. These are a result of portosystemic shunting due to portal hypertension from the obstructed portal vein. On abdominal imaging with ultrasonography or CT, the hepatopetal collaterals of CPVT appear as serpiginous structures, while the main portal vein and/or its branches are not visible.(10)
In our patient, the cause of the isolated bleeding gastric varix is unknown. It occurred in the absence of liver cirrhosis or portal hypertension, and the splenic vein was patent. The partial portal vein thrombosis was fortuitously identified early on CT while working up the cause of the gastric varix. The retrograde pattern of embolisation suggests a preexisting shunt from the left gastric vein to the splenic vein, unlike portosystemic shunts that develop in a background of portal hypertension and give rise to systemic embolisations instead. In view of the decreased portal vein flow on Doppler ultrasonography and the apparent sclerosed isolated gastric varix, we decided to cautiously start our patient on anticoagulation for three months. The role of anticoagulation in this case was to prevent propagation of intravenous thrombus and potential complete portal vein occlusion due to nonlaminar blood flow, generated by the irregular surface of the cyanoacrylate embolus while awaiting the embolus to be endothelialised. The use of anticoagulation had its associated risk of significant bleeding and our patient was closely monitored and advised to return should symptoms of bleeding occur. Close follow-up with Doppler ultrasonography and CT was instituted. In our patient, the emboli resolved uneventfully and CPVT was avoided. To our knowledge, this is the first case with main portal vein embolisation secondary to cyanoacrylate sclerotherapy treated as such.


