Abstract
INTRODUCTION
First trimester screening (FTS) is a validated screening tool that has been shown to achieve detection rates of 84%–90% for trisomies 21, 18 and 13. However, its effectiveness for different maternal ages has not been assessed. The present study aimed to assess the performance of FTS in an Asian population, and to compare its effectiveness in older (≥ 35 years) and younger (< 35 years) women. The potential use of noninvasive prenatal test (NIPT) as a contingent screening test is also examined.
METHODS
Data on cases of FTS performed on singleton pregnancies over a six-year period was collated from two Singapore maternal centres, National University Hospital and Singapore General Hospital. Cases that had a 1:250 risk of trisomy were considered to be screen-positive. Pregnancy outcomes were obtained from birth records or karyotype test results.
RESULTS
From 10,289 FTS cases, we obtained a sensitivity of 87.8%, a specificity of 97.6%, a false positive rate of 2.4% and a false negative rate of 0.06% for the detection of aneuploidy. The overall detection rate for trisomy 21 was 86.5%–85.7% for older women and 87.5% for younger women. The mean number of invasive tests required per case of trisomy 21 was 9.3 in younger women, 8.6 in older women and 13.5 in women with intermediate risk (1:250–1,000).
CONCLUSION
While the performance of FTS was similar in younger and older women, more invasive procedures were required to diagnose trisomy 21 in women with intermediate risk. It may be advantageous to offer contingent NIPT to this group of women to reduce the risk of iatrogenic fetal loss.
INTRODUCTION
First trimester screening (FTS) is a validated screening modality for major chromosomal aneuploidies (i.e. trisomies 21, 18 and 13).(1-3) Using a combination of maternal age, fetal nuchal translucency thickness, maternal levels of serum free β-human chorionic gonadotropin (β-hCG) and pregnancy-associated plasma protein A (PAPP-A), FTS has been shown to achieve a detection rate of 84%–90%, with a false positive rate of 5%.(4-6) Although FTS has been adopted in many countries as the first-line screening for trisomy 21 (i.e. Down syndrome), its effectiveness in women of different ages has not been adequately assessed. A recent observational study reported that FTS is less effective in women aged < 35 years and that a greater number of invasive procedures needs to be performed in this group of women to diagnose a case of trisomy 21.(7) This finding is contrary to reports on other Caucasian populations.(8-10)
If the performance of FTS varies in women of different ages, it is clinically pertinent that these variations are understood. This is especially since the proportion of pregnant women of advanced maternal age, defined as age 35 years and above in most countries, has increased in the past decade. It is unclear whether the widely published detection rates in the literature can be universally applied to women of all ages, especially those of advanced maternal age, as they appear to have a higher risk for fetal trisomy 21.
There has been a growing body of evidence regarding the high negative predictive value of noninvasive prenatal testing (NIPT) using cell-free DNA (cfDNA) and its potential to reduce iatrogenic fetal loss.(11-14) Several countries have already started to recommend the use of NIPT using cfDNA for contingent screening in women who are at a higher risk of a fetus with trisomy 21, including women of advanced maternal age.(15-18) There is, however, a lack of information regarding the potential incremental costs that follow contingent screening, especially in Singapore.
The objectives of the present study were to assess the performance of FTS in an ethnically mixed population for the detection of fetal trisomy 21, and to compare the effectiveness of FTS in women of advanced maternal age (i.e. ≥ 35 years) with that of FTS in women aged < 35 years. We also aimed to determine the incremental cost of detecting one case of trisomy 21 if NIPT was offered to all women who had intermediate- or high-risk scores following FTS.
METHODS
The data used in the present study was derived from two tertiary maternity centres in Singapore – National University Hospital (NUH) and Singapore General Hospital (SGH). All cases of FTS performed in these two centres between January 2006 and December 2011 were collated. At both institutions, FTS was strictly performed between 11+0 and 13+6 weeks of gestation, according to the guidelines established by the Fetal Medicine Foundation (FMF), United Kingdom.(19) All ultrasound assessments of fetal crown-rump length, NT and nasal bone were performed by sonographers who had achieved the appropriate FMF Certificate of Competence. The risks for trisomies 21, 18 and 13 were calculated using an algorithm provided by the FMF. At NUH, karyotyping by amniocentesis or chorionic villus sampling is advised when the FTS risk is 1:250 and above, while at SGH, karyotyping is advised when the risk is 1:300 and above. These patients are considered to be ‘high risk’.
The final study population included only singleton pregnancies and cases in which the pregnancy outcome was known (either via karyotype test results or birth records). All cases were classified as either screen-positive or screen-negative. In the present study, screen-positive cases were identified as those that had a risk greater than 1:250 for trisomy 21, 18 or 13; this criteria was used for all eligible patients. We did not observe any significant difference in the number of invasive procedures performed at the centre that used a cut-off value of 1:300 (i.e. SGH) and the centre that used a cut-off of 1:250 (i.e. NUH). The karyotype test results of all cases where invasive testing was performed were known, as were the delivery outcome of all cases where invasive testing was declined. If a patient was classified as screen-negative after FTS, her status would not be altered even if she opted for elective invasive testing or if invasive testing was performed based on soft ultrasound markers. This is analogous to an intention-to-treat analysis. All delivery outcomes in the screen-negative group were known.
The overall sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of FTS using the risk cut-off value of 1:250 was calculated and compared to its performance when different risk cut-off values were applied. We also compared the performance of FTS for women aged < 35 years and women aged ≥ 35 years at the risk cut-off value of 1:250. To calculate the incremental cost per case of trisomy 21 detected by NIPT, we tabulated the cumulative number of cases screened positive and the number of trisomy 21 cases detected at different risk cut-off values. The cost of NIPT in Singapore was estimated to be around S$1,000. Statistical analysis was performed using the MedCal software (
RESULTS
A total of 10,295 cases of FTS were identified after the inclusion criteria were applied. The ethnicity of the study population was predominantly Chinese and Southeast Asian (n = 9,604, 93.3%) and the mean maternal age was 31.6 ± 4.3 (range 16–47) years. Of the 10,295 women, 2,682 (26.1%) were aged ≥ 35 years. The median gestational age at which FTS was performed was 12+4 (range 11+0 to 13+6) weeks.
The outcome of all cases that underwent FTS is summarised in
Fig. 1
Outcome of the cases that underwent first trimester screening, in National University Hospital and Singapore General Hospital, from 2006–2011.
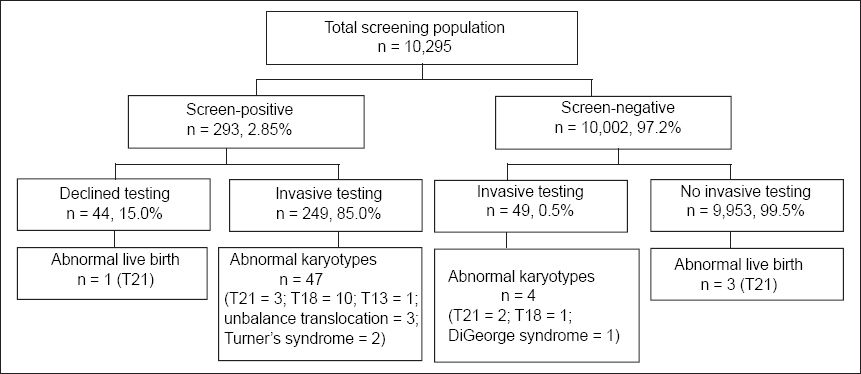
In our study population, 49 of the 10,002 screen-negative cases chose to undergo elective invasive testing. The majority of these 49 cases were motivated to undergo elective invasive testing due to the presence of soft ultrasound markers. Among these 49 cases, abnormal karyotypes were returned in four cases (two cases of trisomy 21, one case of trisomy 18 and one case of DiGeorge syndrome). There were three live births affected with trisomy 21 that were undetected by FTS, giving rise to a total number of six missed trisomies (i.e. false negative) – five trisomy 21 and one trisomy 18. Four of these six babies were born to mothers aged ≥ 35 years and two to mothers aged < 35 years. One miscarriage occurred after amniocentesis and this occurred in a screen-positive patient (the fetus had DiGeorge syndrome). Therefore, the pregnancy loss rate after an invasive procedure was 0.3%. We excluded the case of DiGeorge syndrome from the analysis of FTS performance, since this abnormality was not expected to be detected using FTS, thus resulting in a final sample size of 10,289.
The overall detection rate (sensitivity) of FTS for trisomies 21, 18 and 13 was 87.8%, and the specificity was 97.6%. The false positive rate was 2.4% and false negative rate was 0.06%. The detection rate for trisomy 21 was 86.5% (32 out of 37 cases), while that for trisomy 18 and trisomy 13 was 90.9% (10 out of 11 cases) and 100.0% (1 out of 1 case), respectively. There was a total of 292 invasive procedures performed among all 10,289 cases that underwent FTS. The number of invasive procedures required to make a diagnosis of trisomies 21, 18 or 13 was 6.5 (45 cases detected after 292 invasive procedures), while the number of invasive procedures required to make a diagnosis of trisomy 21 was 8.8 (33 cases detected after 292 invasive procedures).
Table I
Performance of first trimester screening in detecting trisomies 21, 18 and 13 at different selected risk cut-offs (n = 10,289).
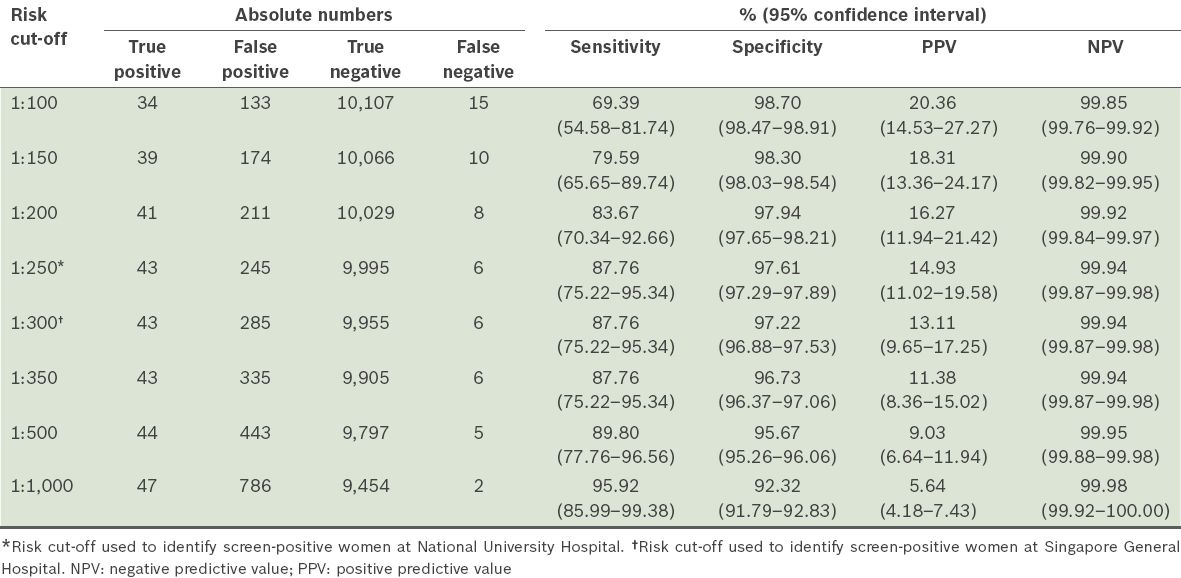
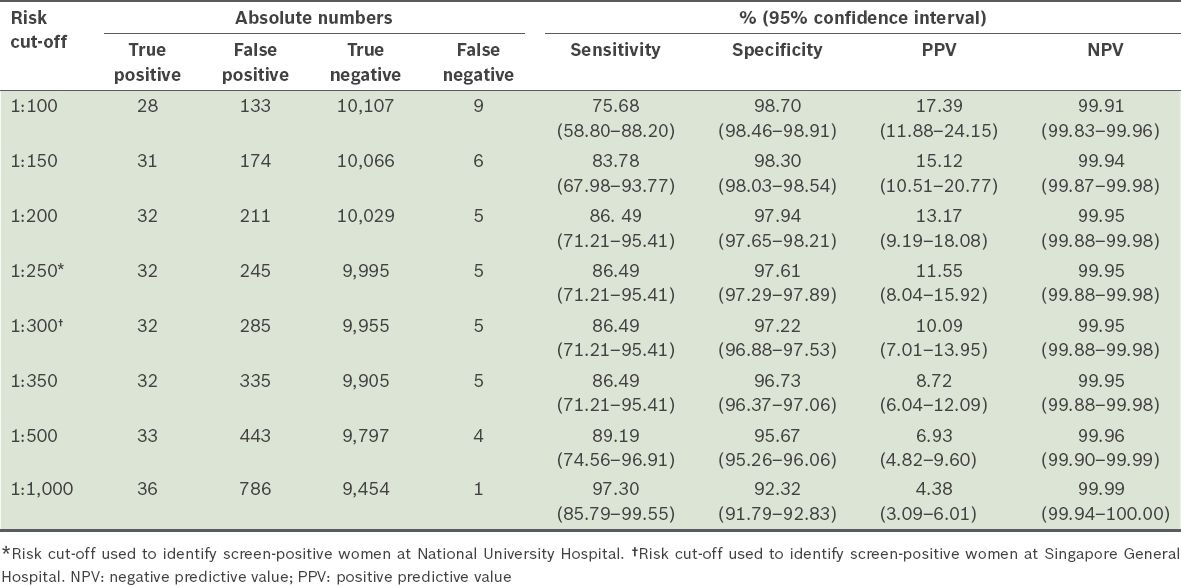
Table II
Performance of first trimester screening in detecting trisomy 21 at different selected risk cut-offs (n = 10,277).
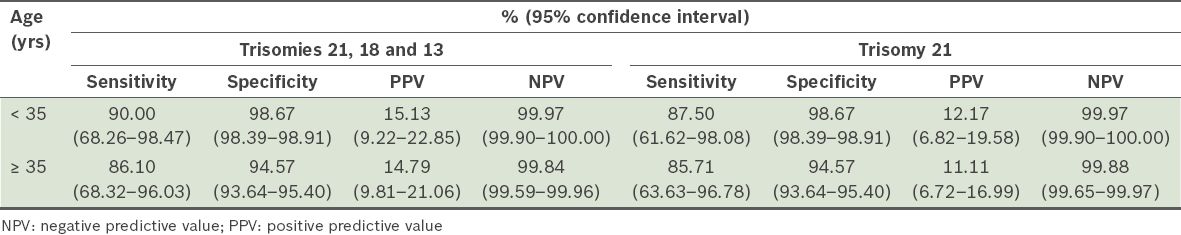
The performance of FTS in women aged < 35 years and ≥ 35 years is summarised in
Table III
Performance of first trimester screening in detecting trisomies 21, 18 and 13, and trisomy 21 (Down syndrome) alone, stratified according to maternal age.
In the present study, there was a total of 833 cases with FTS risk scores that were greater than 1:1,000 (
Table IV
Number and cumulative number of cases, and Down syndrome cases according to different risk cut-off groups.
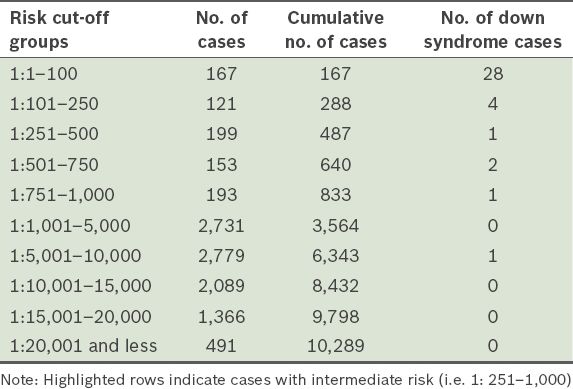
Table V
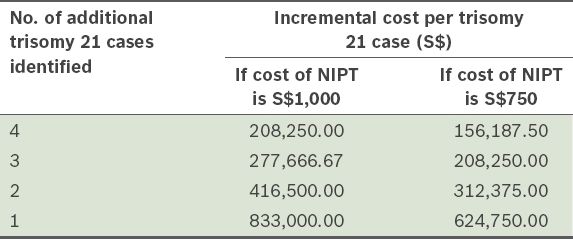
Incremental cost per case of trisomy 21 following noninvasive prenatal test (NIPT) in the first trimester screening intermediate risk group (1:251–1,000).
DISCUSSION
The findings of the present study confirm that FTS is an effective means of screening for trisomy 21 in Southeast Asian populations. The detection rate for trisomy 21 attained in the present study (i.e. 86.5%) is comparable to the rates published for Caucasian(2,3,5,10,20)and Chinese populations.(21-23) This may be attributed to our centres’ strict adherence to the screening guidelines established by the FMF,(19)which includes regular training and auditing of certified antenatal ultrasound sonographers. In addition, FTS attained 90.9% and 100.0% detection rates for trisomies 18 and 13, respectively, in the present study. Both of these aneuploidies are associated with lethal malformations that are incompatible with life.
The overall false positive rate of FTS was 2.4% in the present study. This rate falls below the often-quoted range of 3.5%–5.0%.(5) The pregnancy loss rate after an invasive diagnostic procedure was 0.3% (1/298), which is lower than that often quoted in the literature.(24,25) This may be because only the most experienced providers performed the procedures at the tertiary maternity centres in the present study.
We found that the detection rate for trisomy 21 in women aged ≥ 35 years (85.7%) was slightly lower than that of women aged < 35 years (87.5%). To the best of our knowledge, there is only one study in the literature that assessed the performance of FTS in women of different age groups – an observational study conducted in a Caucasian population.(7)While the authors reported a significantly poorer detection rate in younger women (74.0%), the reported detection rate for older women (87.0%) was comparable to that of the present study. The study was conducted between 2002 and 2008, and it primarily compared women aged < 35 with a selected, much smaller control group of women aged ≥ 35 years. The screening results of that study were also calculated using a different program – the authors used the Perkin Elmer Lifecycle™ algorithm,(7) while we adopted the FMF software.
In the present study, the number of invasive tests per case of trisomy 21 was 9.2 (14 cases detected for 129 procedures performed) in women aged < 35 years and 8.6 (19 cases detected for 163 procedures performed) in women aged ≥ 35 years. FTS was found to have similar efficacy in women aged < 35 years and ≥ 35 years in the present study, which consisted of a large study population. Among women with intermediate risk (1:250–1,000) the number of invasive tests per case of trisomy 21 was 13.5 (2 cases detected for 27 procedures performed). The two cases of trisomy 21 were in women aged > 35 years. Therefore, it was more likely for women with intermediate risk, regardless of age, to be subjected to an invasive procedure, which may result in an increased risk of pregnancy loss.
There was a higher proportion of women in our study population who were aged 35–39 years (23.2%) as compared to the total delivery population at NUH (12.6%) (
Table VI
Comparison of the age distribution of the study population with that of the delivery population in NUH.
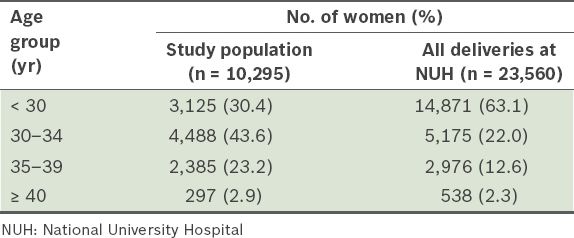
Fig. 2
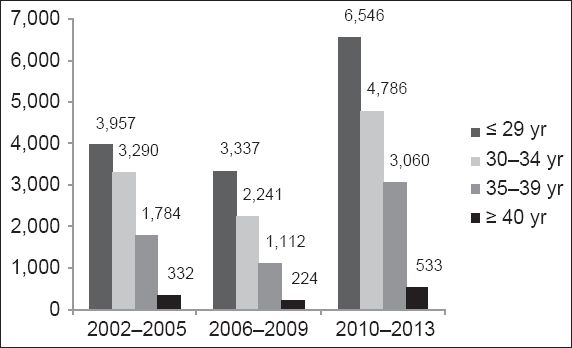
Average number of deliveries in National University Hospital from 2002–2013, over four-year periods and according to the various age groups (n = 27,399).
It may be advantageous to offer the high-risk group NIPT using cfDNA to reduce the risk of iatrogenic fetal loss. Using a combined false positive rate of 0.43% for trisomies 21, 18 and 13,(14) we would expect the number of false positive in the group of women with high-risk screening results (i.e. risk > 1:250) to be reduced from 245 to 2, thus reducing the number of unnecessary invasive procedures by 120-fold. In a study by Gil et al, the authors suggested that NIPT could be offered as a contingent screen following FTS results.(14) They proposed that women with intermediate risk (defined as 1:11–2,500 in that study) could also be offered cfDNA analysis.(14) We suggest that the use of risk scores between 1:251 and 1:1,000 may be a more cost-effective threshold. In the present study, we observed six false negative cases (five trisomy 21 and one trisomy 18). If the uptake of NIPT within the group with a FTS risk of 1:251–1,000 was 100% with a minimum sensitivity of 92% (based on the detection rate for trisomy 13),(14) we would expect to detect an additional four cases of aneuploidy. That is, we would miss one case of trisomy 18 (risk 1:1,066 by FTS) and one case of trisomy 21 (risk 1:8,801 by FTS). Although widening the threshold to 1:251–1,500 would allow identification of the false negative trisomy 18 case, the trisomy 21 case with 1:8,801 risk would still not be detected using contingent screening. As sequencing technology improves and its cost decreases, it is likely that NIPT will be incorporated into routine screening for fetal aneuploidy in all pregnant women in the future.
The present study was not without limitations. One such limitation was the inability to obtain karyotype confirmation for pregnancies that ended in spontaneous miscarriage, and intrauterine fetal demise or termination. In other words, the prevalence of trisomies 21, 18 and 13 may have been underestimated in the present study. Besides that, some studies have shown that using the FTS algorithm to calculate the gestation-specific multiples of the medians (MoMs) of free b-hCG and PAPP-A levels in Chinese populations would result in significantly higher values than if these MoMs were calculated using a Chinese-specific formula.(26,27) If this adjustment was taken into account in the present study, there is a possibility that the screen-positive rate would have been increased. However, as the MoMs of biochemical markers in patients of other ethnicities, which comprised more than 20% of our study population, have not been examined in large cohort studies, the effect of such an adjustment in the risk calculation is still unclear.
The total delivery rate of both tertiary maternity centres is approximately 4,000 per year. About 50% of the patients who were screened at our centres chose to deliver in the same centre. At the moment, although there is no established computerised system, we rely on a closed feedback loop system that requires any trisomy 21 cases delivered outside the institution where screening was performed to be reported back to the screening institution.
To the best of our knowledge, the present study is the first in Singapore to assess the performance of FTS in women of different ages and the incremental costs associated with offering NIPT to women with intermediate or high risk following FTS. From the current published literature, it appears that the performance of FTS in ethnic groups such as Malays has yet to be examined. The high sensitivity and low false positive rates attained in the present study may be attributed to the centres’ high standards of quality control that are in place for first trimester ultrasound examination and their compliance to regular external quality validation by FMF. Robust delivery outcome data was also easily obtained because the databases at both centres were well organised and there was close communication between the centres and their cytogenetic laboratories, which keep records of live births with trisomy 21.
To conclude, the results of the present study provide supporting evidence that clinicians in Singapore should recommend FTS as a first-line screening for trisomy 21, regardless of maternal age. Older women (i.e. aged ≥ 35 years) should be informed that the false positive rate of FTS is higher for them than for younger women. Women who have intermediate risk are more likely to undergo invasive diagnostic testing. These two groups of women should be offered the alternative of NIPT to further stratify their risk.
ACKNOWLEDGEMENT
We would like to acknowledge Ms Dawn Chia from the Fetal Care Centre, National University Hospital, Singapore, for her help in collating the centre’s FTS results.


