Dear Sir,
Ceftriaxone is a commonly prescribed third-generation cephalosporin. The potential risk of biliary pseudolithiasis following the use of ceftriaxone, although very rare, is certainly not limited to the neonatal age group. The clinical course of this seemingly infrequent side effect needs to be recognised in order to avoid multiple blood tests and radiological investigations, which may cause anxiety for patients and parents. We report a unique case of ceftriaxone-induced pseudolithiasis in a previously well five-month-old girl, who received two doses of intravenous (IV) ceftriaxone.
Our patient was diagnosed with left-sided vesicoureteric junction obstruction and Escherichia coli-associated pyelonephritis. She received two doses of IV ceftriaxone (50 mg/kg/dose daily). The was changed to IV co-amoxiclav when the urine culture result was known. Her fever had settled by then and she remained well. On the third day after stopping ceftriaxone, she developed pale stools, which persisted for four days. She had no history of neonatal jaundice and was not on any other medication. On physical examination, she was found to be thriving with no clinical jaundice or hepatosplenomegaly. Liver function tests showed transaminitis (aspartate aminotransferase [AST] 140 [normal range 20–65] U/L and alanine aminotransferase [ALT] 321 [normal range 10–40] U/L) and elevated gamma-glutamyl transferase (GGT) of 814 (normal range 15–110) U/L. Ultrasonography of the hepatobiliary system showed multiple mobile echogenic foci within the gallbladder with moderately thickened gallbladder wall, which suggested biliary sludge/gallstones (
Fig. 1
US images of the hepatobiliary system (a) at presentation shows the gallbladder with multiple mobile echogenic foci suggestive of biliary sludge/gallstones and (b) nine days later shows a collapsed gallbladder with no definite calculi seen within.
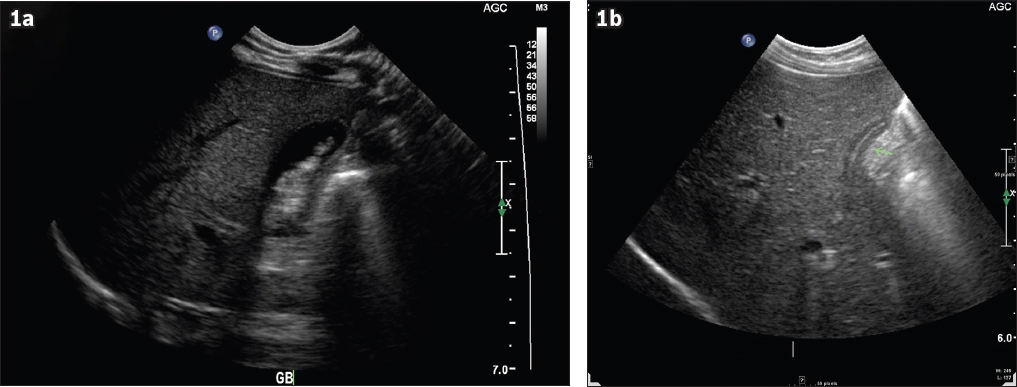
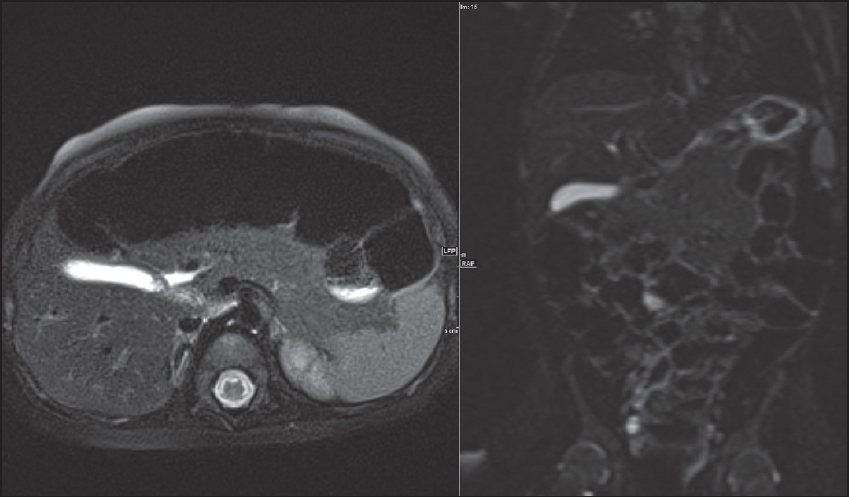
Fig. 2
MR images show a normal gallbladder with no cholelithiasis.
Ceftriaxone-associated pseudolithiasis was first described in 1988 by Schaad et al.(1) Once ceftriaxone is administered, approximately 40% of the administered dose is excreted unmetabolised in the bile, while the rest is excreted in the urine.(2) The potential risk of ceftriaxone causing biliary pseudolithiasis is probably due to its precipitation in the gallbladder.(3) The incidence of ceftriaxone-induced pseudolithiasis was reported to be 25% (n = 11/44) in a study by Papadopoulou et al.(4) In 6 (54%) of these 11 children, pseudolithiasis developed within three days of ceftriaxone administration.(4) Previous studies have not shown any strong evidence with regard to whether ceftriaxone administration in divided doses is associated with reduced biliary sludge formation. A similar result was seen when bolus dosing was compared to slow IV infusion.(3) Previous studies have also shown that pseudolithiasis is more frequent in older children, when ceftriaxone is given at higher doses and for a longer duration of treatment.(3,4) The majority of these children were asymptomatic, although some presented with right-sided abdominal pain and/or abdominal distension.(1,4)
To the best of our knowledge, our patient is the first to present with acholic stools. A recent study suggested that a short period of fasting and bed rest are both associated with biliary pseudolithiasis.(5) Ensuring adequate hydration may help to reduce the occurrence of this side effect. The mean duration to resolution of biliary pseudolithiasis is about 15 days.(6) Cessation of ceftriaxone was the only treatment necessary in all reported cases.(1,3,4) However, one patient was reported to require laparoscopic cholecystectomy subsequent to biliary pseudolithiasis secondary to ceftriaxone treatment.(7) Physicians and radiologists need to be aware of this potential adverse effect so as to avoid unnecessary investigations.
Yours sincerely,


