Abstract
Ketamine is a short-acting anaesthetic agent that has gained popularity as a ‘club drug’ due to its hallucinogenic effects. Substance abuse should be considered in young adult patients who present with severe debilitating symptoms such as lower urinary tract symptoms, even though the use of controlled substances is rare in Singapore. Although the natural history of disease varies from person to person, a relationship between symptom severity and frequency/dosage of abuse has been established. It is important to be aware of this condition and have a high degree of clinical suspicion to enable early diagnosis and immediate initiation of multidisciplinary and holistic treatment. A delayed diagnosis can lead to irreversible pathological changes and increased morbidity among ketamine abusers.
INTRODUCTION
Ketamine is an N-methyl-D-aspartic acid receptor antagonist that is used as a short-acting anaesthetic agent. It has gained popularity as a ‘club drug’ due to its hallucinogenic effects. Ketamine, also known by its street names ‘K’, ‘Special K’, ‘Super K’, ‘Vitamin K’ and ‘Kit Kat’, is sold on the streets in a white crystalline powder or tablet formulation and in an injectable prescription formulation in the clinical setting. It can be ingested, smoked, snorted, or injected intravenously or intramuscularly.
Ketamine has a progressive effect on the user. First, the abuser experiences euphoria, followed by a state of dreamy intoxication, out-of-body experiences, altered sensations and amnesia. Although these addictive effects typically last an hour or less,(1,2) the adverse effects can be long-lasting. Commonly known adverse effects include cardiorespiratory effects, nausea, vomiting, hallucinations and convulsions.(3,4) A less commonly elicited adverse effect is lower urinary tract symptoms (LUTS), which was identified and documented only in the last few years. Studies estimate that 20%–30% of ketamine abusers suffer from LUTS.(1,5) A relationship between symptom severity and frequency/dosage of abuse has been established, and is usually determined by the chronicity of the abuse.(1)
CLINICAL PRESENTATION AND INVESTIGATIONS
We herein illustrate the clinical presentation of four patients (three male and one female aged 21–33 years) who presented with severe intractable LUTS following ketamine abuse. The presenting complaints are shown in
Table I
Overview of clinical presentation of the four patients.

Routine investigations, such as urinalysis and urine culture, typically show sterile urine and occasionally, sterile pyuria,(1) as is the case with all four patients. Early morning urine samples showed negative acid-fast bacilli smear and tuberculosis culture. Given the clinical progression of ketamine-induced cystitis (KC), we suggest an initial assessment of renal function and subsequent follow-up.(2,6) It is believed that on cessation of ketamine abuse, the natural history of disease is not only dependent on the severity and duration of ketamine abuse but also varies from person to person. Approximately one-third of patients have resolved symptoms, one-third continue to have persistent symptoms and one-third experience worsening symptoms.(1) Deterioration in renal function mandates more aggressive treatment.
A voiding diary should ideally be kept, as it allows objective quantification of small bladder capacity and frequent voiding.(6) Ultrasonography of the upper urinary tract may reveal unilateral or bilateral hydronephrosis, circumferential mural thickening with a distended bladder and small bladder capacity (
Fig. 1
US images of the bladder show circumferential mural thickening of the bladder in patient 3.
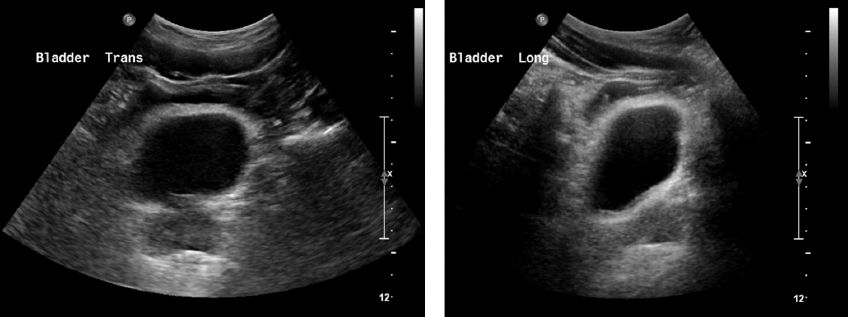
Fig. 2
CT urography image shows (a) gross hydronephrosis of the right kidney and (b) a small shrunken bladder with diffuse wall thickening and perivesical fat stranding in patient 3.
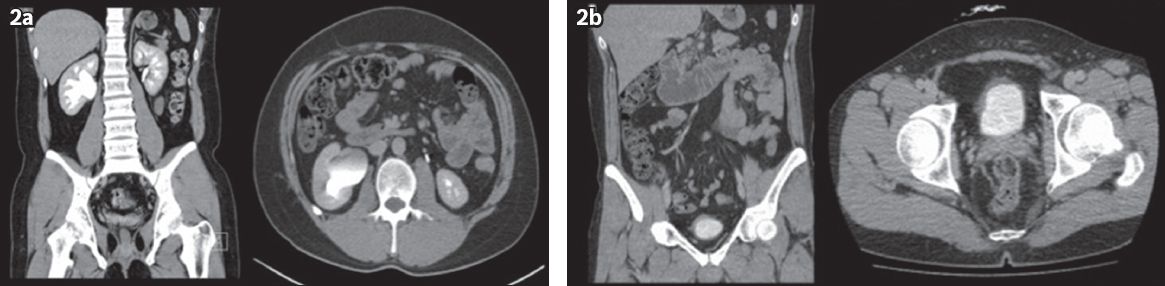
Fig. 3
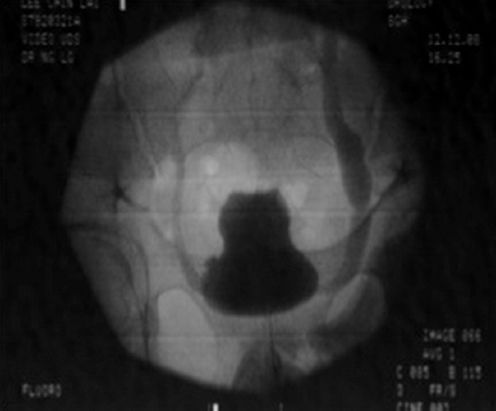
Fluoroscopy images show (a & b) retrograde double-J stent insertion and (c) gross hydronephrosis of the right kidney in patient 2.
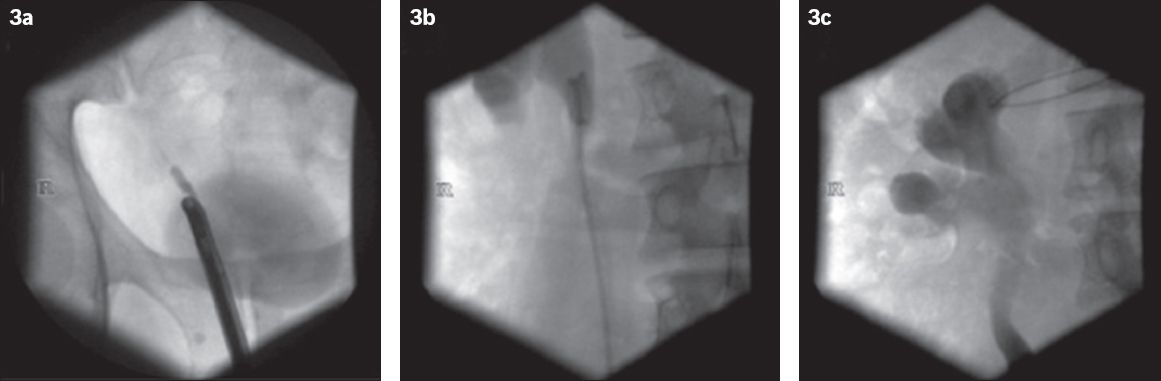
Fig. 4
Video urodynamic study (pressure-flow against time plot) shows small bladder capacity (< 100 mL), with decreased bladder compliance associated with detrusor overactivity in patient 2.
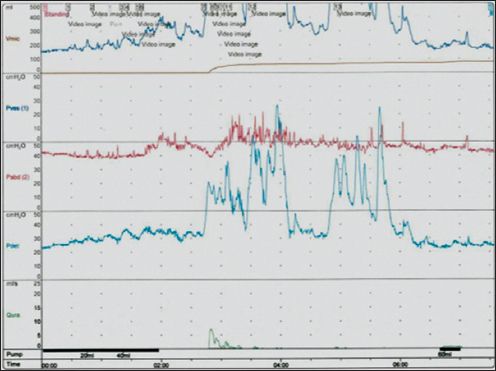
Fig. 5
Fluoroscopy image shows detrusor overactivity with vesicoureteral reflux in patient 3.
Cystoscopic examination is commonly performed under general anaesthesia, as patients are typically unable to tolerate the intense urgency and bladder pain.(6) Findings may be consistent with ulcerative cystitis, revealing inflammation of the urothelial epithelium with glomerulations (described as small discrete submucosal petechial haemorrhages), erythematous congestion, neovascularisation and ulceration (
Fig. 6
Cystoscopy image shows inflammation of the urothelial epithelium with glomerulations, erythematous congestion and neovascularisation.
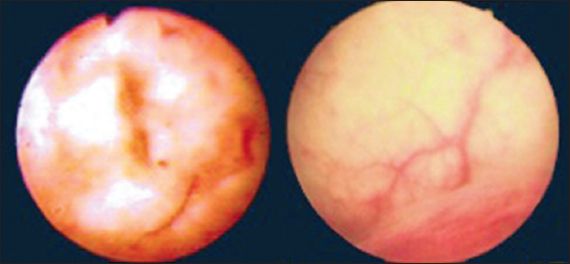
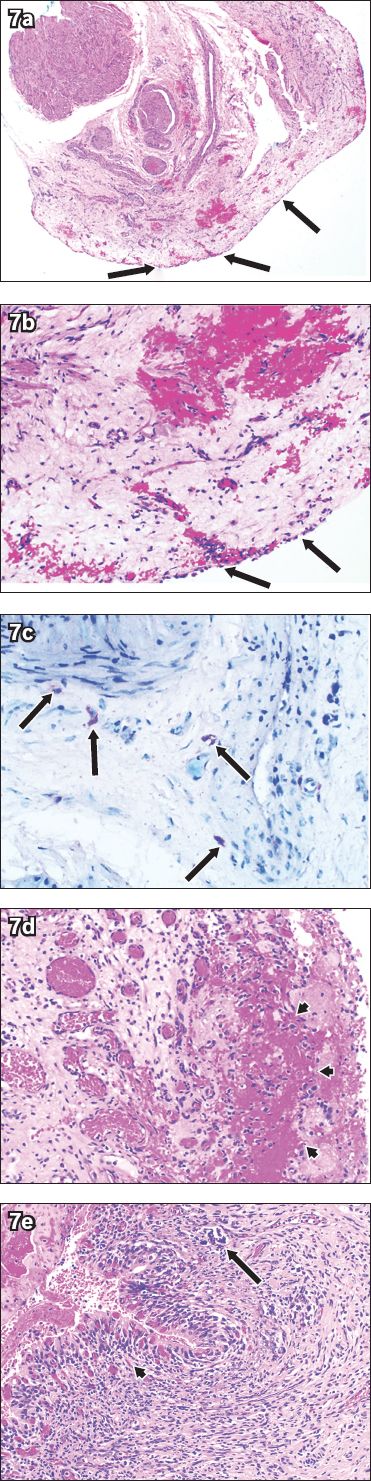
Fig. 7
Photomicrographs of the bladder tissue show (a & b) denudation of the urothelial epithelium (arrows) with oedema and congestion in the lamina propria (Haematoxylin & eosin, × 40 and × 200, respectively); (c) toluidine blue-stained scattered mast cells in the lamina propria, characterised by purple-stained cytoplasmic granules (arrows) (Toluidine blue, × 400); and (d & e) denudation of the urothelial epithelium with adherent haemorrhage and fibrin (arrowheads) and scattered mast cells (arrow) (Haematoxylin & eosin, × 200).
DISCUSSION
Pathogenesis of ketamine-induced cystitis
Ketamine, norketamine and hydroxynorketamine are found in large quantities in the urine of ketamine abusers.(2) Although the exact pathogenesis of KC remains unknown, several mechanisms have been postulated by Chu et al.(5) Firstly, the direct toxic effect of ketamine and its metabolites on interstitial cells of the bladder and renal papillae causes a chronic inflammatory response. In the bladder, this results in submucosal and muscle fibrosis, accounting for dysuria and a contracted bladder. Similarly, in the kidney, this may result in interstitial fibrosis and structural damage, eventually leading to chronic renal insufficiency. Secondly, endothelial injury of microvessels in the urinary system leads to either compromised intrinsic microcirculation or decreased microvascular density in the subendothelium. This could potentially cause bladder ischaemia and subsequently, suprapubic pain and dysuria during bladder filling. Microangiopathy in the renal medulla and the subsequent hypoperfusion of the papilla could then result in tubulointerstitial nephropathy and cortical atrophy of the kidney. Thirdly, urinary ketamine and its metabolites may trigger an autoimmune reaction of the bladder’s urothelial epithelium and submucosa, causing an inflammatory response and scarring, resulting in a poorly compliant, small-capacity bladder.
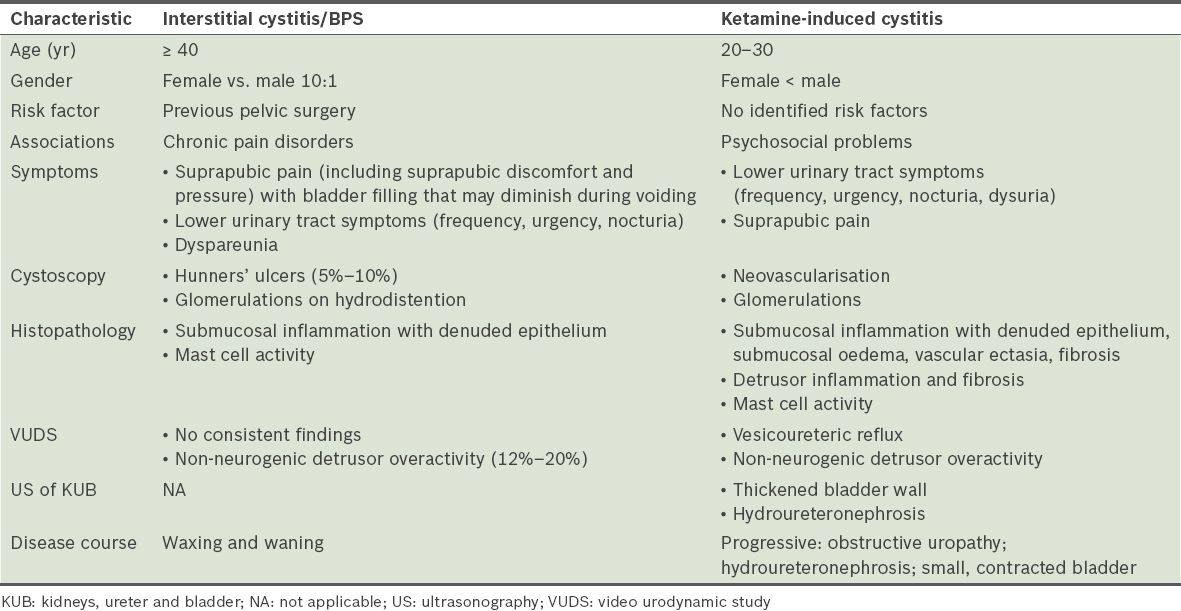
Comparison of ketamine-induced cystitis and interstitial cystitis
Interstitial cystitis (IC) and KC share some similar features in terms of clinical presentation and histological findings. One of these features is bladder pain syndrome (BPS), which is defined by the International Continence Society as “the complaint of suprapubic pain related to bladder filling, accompanied by other symptoms such as increased daytime and night-time frequency, in the absence of proven urinary infection or other obvious pathology.”(8) IC is defined as BPS with typical cystoscopic and histologic features;(8) it is most commonly diagnosed in patients over the age of 40 years, with a female predilection of 10:1. It is associated with a high rate of prior pelvic surgery and other systemic chronic pain disorders, such as irritable bowel syndrome, chronic fatigue syndrome and fibromyalgia. However, due to the different pathophysiology of KC and IC, they each have a markedly different natural history of progression. KC affects both the upper and lower urinary tracts, leading to a small, contracted bladder and obstructive uropathy, whereas IC is a chronic bladder condition that follows a waxing and waning course.(8)
Table II
Comparison of the characteristics of ketamine-induced cystitis and interstitial cystitis/bladder pain symdrome (BPS).
Treatment
Ketamine detoxification is paramount in the treatment of ketamine abuse. However, the effectiveness of this treatment is dependent on the severity and duration of ketamine abuse.(1) Although early recognition and diagnosis may prevent irreversible pathological changes,(6) there is no single definitive management pathway. The key to effective treatment is having an individualised approach with an emphasis on compliance.
Pharmacotherapy for KC is similar to that for IC. Pain modulators such as tricyclic antidepressants (e.g. amitriptyline) act by inhibiting the presynaptic reuptake of serotonin and noradrenaline, hence modulating the transmission of nociceptive stimuli. Anti-inflammatories such as H2 receptor antagonists (e.g. cimetidine) act as competitive inhibitors of histamine, which is the key mediator of mast cells. Other therapeutic strategies involve approaches aimed at repairing the damaged urothelial epithelium. Pentosan polysulfate (Elmiron®), a low-molecular-weight, heparin-like compound, acts by coating the bladder lining and repairing the glycosaminoglycan layer of the damaged urothelial epithelium. Intravesical instillations of pentosan polysulfate and hyaluronic acid have been used, as they are thought to function as a protective barrier on the urothelial epithelium.
Surgery is indicated in cases where conservative methods have failed. Intravesical botulinum toxin A injection and cystodistension have been used to increase functional bladder capacity and reduce pain. Percutaneous nephrostomy or retrograde double-J stent insertion has been performed to alleviate hydronephrosis and deteriorating renal function. Subtotal cystectomy and augmentation cystoplasty have also been performed in refractory cases to improve renal function and quality of life.
Lastly, a multidisciplinary approach involving medical social workers, psychiatrists, continence/urology nurse-clinicians and urologists should be established as many of these patients have additional psychosocial problems in addition to ketamine dependence. A holistic approach would be more effective in initiating and sustaining ketamine cessation.
Local context
The Central Narcotics Bureau in Singapore noted that in 2013, 4.27 kg of ketamine was seized. This was up 10% from 2012, when 3.89 kg was seized.(9) As a fear of criminal prosecution prevents patients from volunteering a history of substance abuse, directed questions are essential. Although substance abuse is uncommon in Singapore, ketamine-associated urinary tract dysfunction should be considered in young adults presenting with severe intractable LUTS.
CONCLUSION
The history of ketamine abuse should be specifically elicited. Delayed diagnosis can lead to irreversible pathological changes and increased morbidity; hence, early urology referral is advocated.


