Abstract
INTRODUCTION
Type 2 diabetes mellitus (T2DM), the tenth leading cause of death in Hong Kong, has a prevalence of approximately 10%. Sodium-glucose co-transporter-2 (SGLT2) inhibitors lower glycated haemoglobin (HbA1c) levels in T2DM patients via a non-insulin-dependent mechanism of action, but real-world data is limited, particularly for Chinese patients.
METHODS
A retrospective single-centre study was performed among Chinese patients with T2DM who were prescribed SGLT2 inhibitor therapy in Hong Kong. Changes in HbA1c levels, body weight, systolic and diastolic blood pressure, estimated glomerular filtration rate (eGFR), lipid profiles and adverse events were observed for patients who completed at least one follow-up visit during the study period.
RESULTS
Overall, 100 patients were included, and 53 patients attended an additional final visit. By the final visit, SGLT2 inhibitor therapy had significantly decreased HbA1c levels (change [Δ] 0.31%, 95% confidence interval [CI] −0.11% to −0.51%, p < 0.001), body weight (Δ −4.59 kg, 95% CI −3.75 to −5.54 kg, p < 0.001) and systolic blood pressure (Δ −5.72 mmHg, 95% CI −1.72 to −9.72 mmHg, p < 0.001) from baseline. No significant change in eGFR or lipid profiles was observed, except for a significant reduction in high-density lipoprotein cholesterol (Δ −0.09 mmol/L, 95% CI −0.16 to −0.02 mmol/L, p < 0.05). Adverse events were consistent with previous reports for SGLT2 inhibitors, apart from appetite loss associated with canagliflozin.
CONCLUSION
The real-world efficacy and safety profile of SGLT2 inhibitors in Chinese patients was comparable to that reported in Phase III clinical trials, with the exception of appetite loss among patients who received canagliflozin.
INTRODUCTION
Diabetes mellitus is a global epidemic affecting 422 million people worldwide, or approximately 8.5% of the global population,(1) and is associated with an increased risk of cardiovascular disease, kidney failure, retinopathy and neuropathy. In 2012 alone, diabetes mellitus was designated as the cause of death for 1.5 million people worldwide, while elevated blood glucose was responsible for an additional 2.2 million deaths.(1) These statistics are also reflected in Hong Kong, where over 580,000 people aged 20–79 years, or approximately 10% of its adult population, have type 2 diabetes mellitus (T2DM), which is listed as the tenth leading cause of death.(2,3)
T2DM is characterised by hyperglycaemia resulting from resistance to insulin – the hormone that stimulates glucose uptake in the liver, muscles and adipose tissue – and/or a relative insulin deficiency, especially in patients with obesity.(4) The degree of hyperglycaemia in patients with T2DM has, in turn, been correlated with several cardiovascular and clinical outcomes.(5)
Therefore, the primary aim of treatment for patients with T2DM is to maintain glycaemic control with lifestyle modification and pharmacological interventions. While metformin is considered to be the first-line treatment for the pharmacological treatment of T2DM, the relationship between insulin-dependent pharmacological interventions and disease progression in T2DM is complex.(4) Oral antidiabetic drugs (OADs) with an insulin-dependent mechanism of action, such as sulphonylureas (SUs) and incretin mimetics (dipeptidyl peptidase-4 [DPP4] inhibitors and glucagon-like peptide-1 agonists), do not address the underlying insulin resistance, and incretin mimetics are also associated with gastrointestinal adverse events, such as diarrhoea and nausea and, in rare cases, pancreatitis.(4) Furthermore, SUs, thiazolidinediones (TZDs) and exogenous insulin are also associated with weight gain. Given that SUs also stimulate the release of insulin independently of the presence of glucose, both SUs and exogenous insulin are also associated with an increased risk of hypoglycaemia, which, in turn, has been linked to an increased risk of major cardiovascular events among patients with T2DM.(4,6)
In contrast, sodium-glucose co-transporter-2 (SGLT2) inhibitors are a new class of OADs offering a non-insulin-dependent mechanism of action by inhibiting the reabsorption of glucose in the glomerular filtrate in the kidneys, allowing excess glucose to be excreted in the urine.(7) This class of drugs has been shown to be efficacious in reducing glycated haemoglobin (HbA1c) levels in patients with T2DM, while also offering the additional benefit of weight loss, blood pressure reduction and increased high-density lipoprotein cholesterol levels, independent of a patient’s degree of b-cell function or insulin sensitivity.(8) Furthermore, recent data not only suggests that the efficacy of SGLT2 inhibitors observed during Phase III clinical trials is replicated in a real-world setting, but also that this class of drugs may be more efficacious than DPP4 inhibitors.(9,10)
While SGLT2 inhibitors require sufficient renal function to be efficacious, no adverse impact on renal function has been reported within this drug class. In fact, it has been suggested that SGLT2 inhibitors may have a nephroprotective effect by reducing serum uric acid levels, tubular glucose toxicity and diabetes mellitus-related hyperfiltration, as evidenced by reduced albuminuria in patients who were administered SGLT2 inhibitors in combination with renin-angiotensin system blockers.(11) Likewise, the first prospective cardiovascular outcomes study for a SGLT2 inhibitor, the EMPA-REG OUTCOME study of empagliflozin, reported a significant reduction in the risk of major cardiovascular events compared with a placebo, although it is not yet clear whether this is a class effect, as prospective cardiovascular outcome studies for dapagliflozin and canagliflozin are ongoing.(12) Furthermore, SGLT2 inhibitors are associated with a low risk of hypoglycaemia, and the most commonly observed adverse events, such as urinary tract infections (UTIs) and genital infections, are generally considered to be mild to moderate in severity and respond to standard treatment.(13)
However, real-world patterns of usage after a drug receives marketing approval are often different from those reported in clinical trials and are shaped by local policy and differing population characteristics, such as polypharmacy and comorbidities, when compared with the defined inclusion/exclusion criteria used in clinical trials, as was observed in the Association of British Clinical Diabetologists nationwide exenatide audit, which found that approximately 40% of exenatide prescriptions were issued off-label, as an add-on to insulin therapy.(14) Therefore, the current study aimed to assess the efficacy and safety of SGLT2 inhibitor therapy in a real-world population of patients of Chinese ethnicity with T2DM.
METHODS
The current study was a single-centre retrospective observational study of 100 patients with T2DM enrolled at a private specialist diabetes clinic comprising four physicians in the Special Administrative Region of Hong Kong, which was conducted as part of a regular clinical audit. Patients enrolled in the study were required to be of Chinese ethnicity, to be taking prescribed SGLT2 inhibitor therapy during an initial enrolment visit that occurred between 1 February 2015 and 30 November 2015, and to have attended at least one follow-up visit during this period. The SGLT2 inhibitor administered (canagliflozin, dapagliflozin or empagliflozin) was chosen at the physician’s discretion. Most patients were followed up every 8–12 weeks at each individual physician’s discretion as part of routine clinical practice, as there was no prespecified standardised follow-up schedule. The visit closest to and within three months was designated as the first follow-up visit and the last available visit after three months was designated as the final visit. As this was a retrospective observational study conducted as part of a clinical audit, written informed consent was not obtained.
As part of the routine clinical management of patients with T2DM, body weight and blood pressure were assessed at every visit, while HbA1c was monitored every 8–12 weeks. Lipid profiles were assessed every six months. Renal function tests were scheduled annually, with optional renal function tests performed at the physician’s discretion after initiating SGLT2 inhibitor therapy. The methodology for estimating glomerular filtration rates was also chosen at the physician or laboratory’s discretion.
Patients were routinely screened for known adverse events associated with the SGLT2 class of OADs, including UTIs, vaginitis, balanitis and postural hypotension. Individual patient data relating to clinical history, concomitant drug utilisation, anthropometric parameters, clinical parameters, biochemical parameters and adverse events were extracted from the case notes of patients.
Data is presented as mean ± standard deviation. Analysis of variance and post-hoc paired t-tests were performed to compare treatment efficacy. Regression analysis was used to explore relationships between baseline characteristics and treatment efficacy. All statistical analyses were performed using SPSS version 14 (SPSS Inc, Chicago, IL, USA). All analyses were exploratory.
RESULTS
The demographic and baseline clinical characteristics of the 100 patients included in this study are presented in
Table I
Patient demographics and clinical characteristics at baseline.
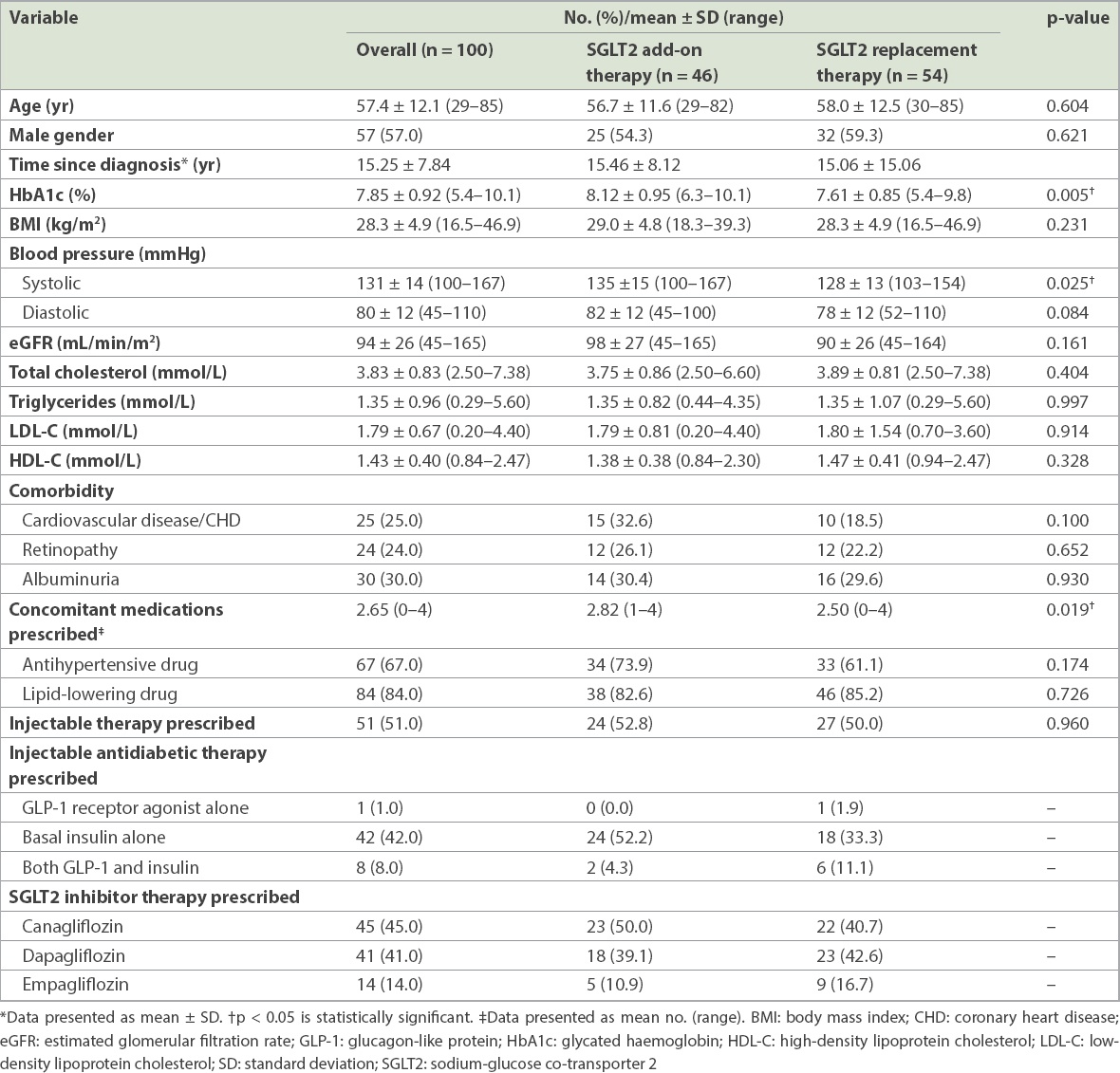
As might be expected, patients who were administered SGLT2 inhibitor add-on therapy were also prescribed a significantly greater number of concomitant medications (p = 0.019). A majority of patients were prescribed SGLT2 inhibitor to improve glycaemic control (76.0%); however, for 25.9% (n = 14) of patients from the SGLT2 inhibitor replacement therapy group, this was because of adverse events after previous medication (
Table II
Reasons for prescribing SGLT2 inhibitor therapy.
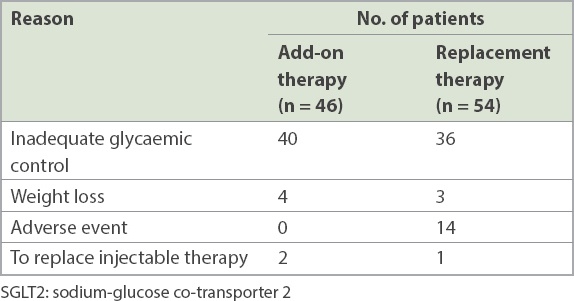
Patients were followed up for a mean duration of 22.7 weeks following the index visit, with a mean duration of 9.9 weeks between the index and first follow-up visits. Data was also available for 53 patients who attended a final visit following the first follow-up visit (add-on therapy, n = 28, replacement therapy, n = 25).
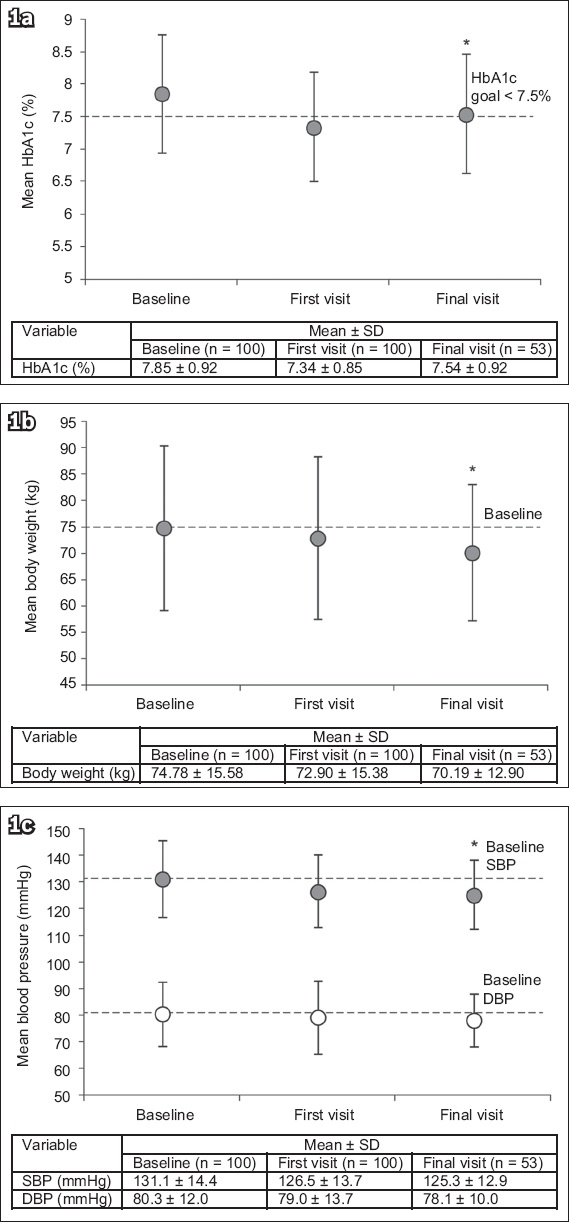
SGLT2 inhibitor therapy significantly decreased HbA1c levels by 0.31% (95% confidence interval [CI] −0.11% to 0.51%, p < 0.001) between the baseline and final visits (
Fig. 1
Charts show overall changes from baseline to final follow-up visit in (a) HbA1c; (b) body weight; and (c) SBP and DBP levels. *p < 0.001 was statistically significant. DBP: diastolic blood pressure; HbA1c: glycated haemoglobin; SBP: systolic blood pressure; SD: standard deviation
No significant difference in the changes in HbA1c levels was observed at the final visit between patients who were administered oral agents alone and those who were administered injectables, or in patients who were administered 1–2 compared to ≥ 3 antidiabetic agents. Likewise, no significant difference was observed between the efficacy of empagliflozin, dapagliflozin or canagliflozin. Regression analyses did not identify any relationship between SGLT2 inhibitor therapy efficacy and body mass index (BMI) or estimated glomerular filtration rate (eGFR).
Significant weight loss was reported at the final visit after initiating SGLT2 inhibitor therapy (Δ −4.59 kg, 95% CI −3.75 to 5.54 kg, p < 0.001 vs. baseline;
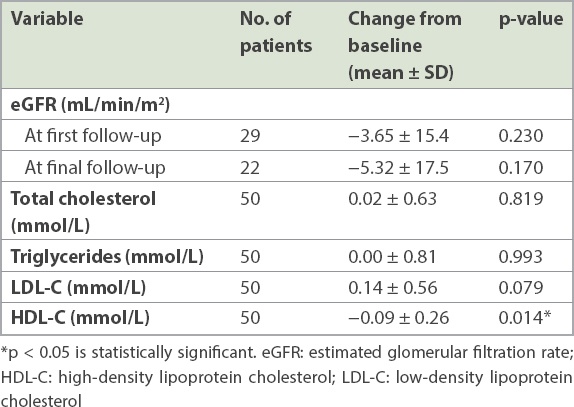
No significant changes in eGFR or lipid profiles were observed, except for a significant reduction in high-density lipoprotein cholesterol (Δ −0.09 mmol/L, 95% CI −0.02 to −0.16 mmol/L, p < 0.05;
Table III
Key findings on renal function and lipid profiles at the final follow-up visit.
Adverse events reported by patients who were administered SGLT2 inhibitors were consistent with those previously reported. At the first visit, five patients had developed UTI and 16 patients reported vaginitis or balanitis. Of the five patients experiencing poor appetite, all were administered canagliflozin. At the final visit, no UTIs were reported, while six patients had vaginitis or balanitis. As at the first follow-up visit, the two patients experiencing poor appetite were both administered canagliflozin.
Seven patients discontinued SGLT2 inhibitor treatment after the first visit, while three additional patients had discontinued therapy at the final visit. Four patients discontinued treatment due to vaginitis or balanitis, while other patients discontinued treatment due to poor appetite and excessive weight loss (n = 3), malaise (n = 1), lack of efficacy (n = 1) and no longer needing treatment because of bariatric surgery (n = 1).
DISCUSSION
The current study presented real-world data of Chinese patients with T2DM who were treated with SGLT2 inhibitors, indicating a comparable efficacy and safety profile when compared with that reported in Phase III clinical trials. In particular, this real-world cohort included a high number of patients with advanced or complicated disease that was associated with diabetes mellitus-related comorbidities and concomitant medications, and required treatment with multiple antidiabetic agents, including injectables. Notably, this study included patients who had been prescribed one of the three SGLT2 inhibitor therapies approved in Hong Kong at the time of this study: canagliflozin, dapagliflozin and empagliflozin. Prior to the study, to our knowledge, published data relating to the use of SGLT2 inhibitors to treat Chinese patients with T2DM was limited to a small study of dapagliflozin monotherapy in drug-naive patients.(15)
The efficacy of SGLT2 inhibitors appeared to be greater when they were prescribed as an add-on therapy rather than replacement therapy, although this was not unexpected given that the efficacy of antidiabetic therapies in improving glycaemic control is known to be dependent on baseline HbA1c levels.(16) Furthermore, clinical trials have generally demonstrated the use of SGLT2 inhibitors as an add-on therapy in patients requiring improved glycaemic control and not as a replacement therapy for those who have adequate glycaemic control but are intolerant to their existing regimen. However, SGLT2 inhibitors offered the additional benefits of weight loss and lower blood pressure as both add-on and replacement therapies.
In contrast to a meta-analysis of clinical trials investigating SGLT2 inhibitor therapy in patients with T2DM, efficacy in the current study was not correlated with patient BMI and a significant decrease in high-density lipoprotein cholesterol levels was observed, as opposed to the previously reported increase.(8) In addition, no correlation was found between efficacy and eGFR, suggesting that SGLT2 inhibitor therapy may be suitable for a broader range of patients than is defined by the current eGFR-dependent approved indications.
However, as was expected from earlier reports, SGLT2 inhibitor therapy was associated with an increased prevalence of UTIs and genital infections in our study, although all UTIs had cleared between the first and final follow-up visits. Interestingly, loss of appetite reported by some patients who were administered canagliflozin does not appear to have been reported previously. As canagliflozin, but not dapagliflozin or empagliflozin, also inhibits sodium-glucose co-transporter-1 (SGLT1),(17) which is expressed in the intestines, SGLT1 inhibition offers a hypothetical mechanism for this adverse event, and its initial reporting in our Chinese population warrants further investigation.
This study had several limitations due to its retrospective analysis of data, which was derived from a small sample at a single specialist centre where all patients were being treated by one of four physicians. This raises the risk of bias in the results reported, given the exploratory nature of all analyses performed. The applicability of this data in a primary care setting may also be limited. Furthermore, the follow-up interval and pattern of assessment were at the treating physician’s discretion, so data from the final follow-up visit was not available for all patients, particularly with regard to some laboratory assessments, such as renal function tests.
In conclusion, the efficacy and safety profile of SGLT2 inhibitors prescribed to Chinese patients with T2DM, observed in the real world, was comparable to that reported in Phase III clinical trials, with the exception of the newly reported adverse event of appetite loss among patients administered canagliflozin. The current study also supports the use of SGLT2 inhibitors to treat a broad range of patients with T2DM encountered in clinical practice, including those with advanced or complicated disease.
ACKNOWLEDGEMENTS
This review was funded by AstraZeneca Hong Kong Limited, Hong Kong. Medical writing support was provided by Blair Hesp, PhD, CMPP, of MIMS (Hong Kong) Limited and funded by AstraZeneca.


