Abstract
INTRODUCTION
The antinuclear antibody (ANA) test is a screening test for systemic autoimmune rheumatic disease (SARD). We hypothesised that the presence of anti-DFS70 in ANA-positive samples was associated with a false-positive ANA test and negatively associated with SARD.
METHODS
A retrospective analysis of patient samples received for ANA testing from 1 January 2016 to 30 June 2016 was performed. Patient samples underwent ANA testing via indirect immunofluorescence method and anti-DFS70 testing using enzyme-linked immunosorbent assay.
RESULTS
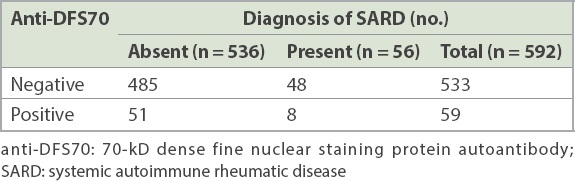
Among a total of 645 ANA-positive samples, the majority (41.7%) were positive at a titre of 1:80. The commonest nuclear staining pattern (65.5%) was speckled. Only 9.5% of ANA-positive patients were diagnosed with SARD. Anti-DFS70 was found to be present in 10.0% of ANA-positive patients. The majority (51/59, 86.4%) of patients did not have SARD. Seven patients had positive ANA titre > 1:640, the presence of anti-double stranded DNA and/or anti-Ro60. The presence of anti-DFS70 in ANA-positive patients was not associated with the absence of SARD (Fisher’s exact test, p = 0.245).
CONCLUSION
The presence of anti-DFS70 was associated with a false-positive ANA test in 8.6% of our patients. Anti-DFS70 was not associated with the absence of SARD.
INTRODUCTION
The antinuclear antibody (ANA) test, performed using indirect immunofluorescence assay (IFA), is the gold standard screening laboratory test for the assessment of systemic autoimmune rheumatic disease (SARD).(1,2)
The origin of the ANA test is closely related to the lupus erythematosus cell, first reported in 1948(3) and found in 25 patients with systemic lupus erythematosus (SLE).(4) A serum circulating factor in SLE patients was suspected to have triggered neutrophil phagocytosis of cell nuclei. Holborow et al, in 1957,(5) demonstrated that this circulating factor had affinity for human cell nuclei. Today, we know that these circulating factors in SLE patients correspond to autoantibodies to double-stranded DNA (dsDNA), Smith (Sm), ribonucleoprotein (RNP), Ro and La.(4) Using the HEp-2 (human epithelial type 2) cell line for ANA IFA, these autoantibodies produce homogenous fluorescent patterns for dsDNA and speckled patterns for Sm, RNP, Ro and La.(1)
Originally intended as a screening test for SLE, the ANA test has now been applied to other SARDs, including Sjögren’s syndrome (SS), systemic sclerosis and the idiopathic inflammatory myopathies. Its prime advantage as a screening test is to direct specific autoantibody testing based on the nuclear or cytoplasmic staining pattern.(1) For example, a speckled nuclear staining pattern on ANA, in the correct clinical context, would prompt an evaluation for SS, anti-Ro and anti-La. A nucleolar staining pattern has been associated with anti-Scl70 (also called anti-topoisomerase I), which is present in patients with systemic sclerosis. The ANA test has also been adopted for evaluation of other organ-specific diseases deemed to be autoimmune in nature. Autoimmune hepatitis is one such example, wherein the presence of ANA and other autoantibodies is integrated into the diagnostic criteria.
The association of ANA staining patterns with disease-specific autoantibodies is well known among physicians, rheumatologists and immunologists alike. However, what is less commonly recognised is the presence of a dense fine nuclear staining (DFS) pattern seen on laboratory investigations. This DFS pattern, characterised by irregularly distributed, fine-granular fluorescence of the nuclei in the interphase and metaphase chromatin,(6) is codified as AC-2 by the International Consensus on ANA patterns,(7) with AC-1 being the homogenous nuclear staining pattern. The first study referencing the DFS nuclear staining pattern was published in 1994,(8) where Ochs et al described this staining pattern in over one-third of sera from 96 patients with interstitial cystitis. Ochs, in a separate collaboration, went on to show that the target antigen for DFS nuclear staining was a 70-kD protein(9) – thus, the nomenclature DFS70. In this earlier study, anti-DFS70 was identified in 29.7% of patients with atopic dermatitis and 16% of patients with asthma. DFS70 protein is identical to a transcriptional coactivator p75, also known as the lens epithelium-derived growth factor (LEDGF) protein. On a cellular level, DFS70/LEDGFp75 is a pro-survival factor that confers resistance to apoptosis induced by cell stress.(10) DFS70/LEDGFp75 is also involved as a cofactor in HIV (human immunodeficiency virus) replication.(11) The exact immunological role of DFS70 is not well understood; experts suggest that anti-DFS70 is an epiphenomenon of systemic inflammation.(6)
Over the past two decades, studies have described the association of anti-DFS70 with a spectrum of chronic inflammatory disorders. Yet, it has also been studied and hypothesised to be associated with healthy individuals with a positive ANA. The possible association with non-SARD in patients with anti-DFS70, the significant proportion of false-positive ANA with anti-DFS70 positivity and the increasingly widespread adoption of the ANA test in other medical specialties(12) have generated much interest in the utility of the anti-DFS70 assay for the clinician, especially among rheumatologists. A significant proportion of patients referred to the rheumatology clinic may have a positive ANA test, performed as a result of suggestive symptoms (such as arthralgia, fatigue or photosensitive rash). Evolving or early SARD is often the clinical concern necessitating rheumatologist review and subsequent laboratory investigations to ascertain disease or non-disease. The burden of repeat outpatient visits and testing represents a significant resource strain. Thus, the ability to discern between disease and non-disease states using a simple serologic test is a cost-effective and valuable one.
Our study aimed to ascertain the local prevalence of anti-DFS70 in a tertiary hospital setting in Singapore. We aimed to test the hypothesis that the presence of anti-DFS70 in ANA IFA-positive samples would be associated with a false-positive ANA test and negatively associated with SARD. We intended to adopt anti-DFS70 assay for routine clinical use if results from our study were positive, given the potential reduction in clinical patient load for the rheumatology outpatient clinic.
METHODS
We performed a retrospective analysis of patient samples received at the Clinical Immunology Laboratory, Tan Tock Seng Hospital (TTSH), Singapore, for ANA testing from 1 January 2016 to 30 June 2016.
All patient samples underwent ANA testing via IFA using Hep 2010 test kit (EUROIMMUN AG, Lüebeck, Germany). Diluted patient sera samples were incubated for 30 minutes with human epithelial cells followed by a washing step and allowed another 30 minutes of incubation time with fluorescein isothiocyanate-labelled antihuman immunoglobulin G (IgG). The incubated slides were then washed again. Specific antibodies bound to antigen would be retained, and slides were mounted to be read by a technician on a Leica LED fluorescence microscope (DM 1000 LED, Leica Microsystems, Shanghai, China). Nuclear staining pattern and positive titre were recorded. ANA testing was reported as positive in the presence of nuclear staining pattern and at a titre of at least 1:80.
All ANA-positive samples were then subjected to anti-DFS70 testing via enzyme-linked immunosorbent assay (ELISA; EA 159z; EUROIMMUN AG). Euroimmun anti-DFS70 test kit was used with Grifols Triturus immunoassay processor (Triturus, Diagnostic Grifols SA, Barcelona, Spain). Diluted patient sera samples were incubated in polystyrene microplate wells coated with purified antigen for 30 minutes, followed by antihuman IgG conjugate for 30 minutes, and finally, 15 minutes with TMB (3,3’,5,5’-tetramethylbenzidine) substrate solution. The wells were washed between these steps to remove unbound antibodies. The assay was stopped with sulphuric acid. Optical density was obtained at a wavelength of 450 nm, with 620 nm as reference. Sample concentration was obtained from a standard curve linear plot measured for the three calibrators against their corresponding units. Qualitative results were obtained by calculating a ratio of the extinction value of the control or patient sample against the extinction value of the calibrator. A ratio greater than or equal to 1.0 was considered positive.
Applying the PICO (Patient, Interest, Comparator, Outcome) framework, the ‘patient’ group included all patients receiving medical care at TTSH, either in the acute hospital wards or at the specialist outpatient clinics, for whom a clinician had requested ANA testing. The ‘interest’ group referred to patients who were ANA positive and anti-DFS70 positive. The ‘comparator’ group referred to patients who were ANA positive and anti-DFS70 negative. The ‘outcome’ assessed was an eventual diagnosis of SARD. Clinical diagnosis was obtained from the electronic medical records of the referring clinician, who was generally from the clinical services of the rheumatology, immunology, nephrology, neurology, gastroenterology and internal medicine specialities. Descriptive statistics were mainly used to present the data. For the cross-table analysis, Fisher’s exact test was used for evaluating the association of anti-DFS70 with the absence of a diagnosis of SARD. This study was approved by the National Healthcare Group Domain Specific Review Board (reference number 2016/00948).
RESULTS
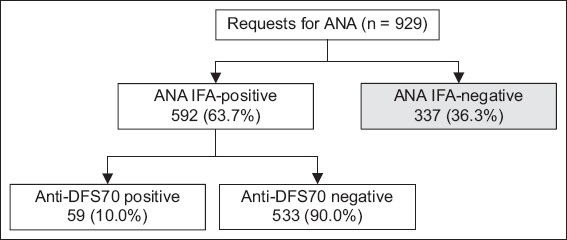
A total of 929 patients (mean age 57 [range 18–98] years) underwent ANA testing during the study period. There were 563 (60.6%) women and 366 (39.4%) men in the cohort. The majority of patients were of Chinese ethnicity (n = 716, 77.1%). There were relatively equal numbers of Indian (n = 81, 8.7%) and Malay (n = 85, 9.1%) patients. There were 645 (69.4%) ANA-positive samples, of which 53 (8.2%) were excluded owing to insufficient sera. There were 337 (36.3%) ANA-negative samples. A total of 592 ANA-positive samples were included in the analysis (
Fig. 1
Flowchart shows results of ANA and anti-DFS70 testing. ANA: antinuclear antibody; IFA: indirect immunofluorescence assay; anti-DFS70: 70-kD dense fine nuclear staining protein autoantibody
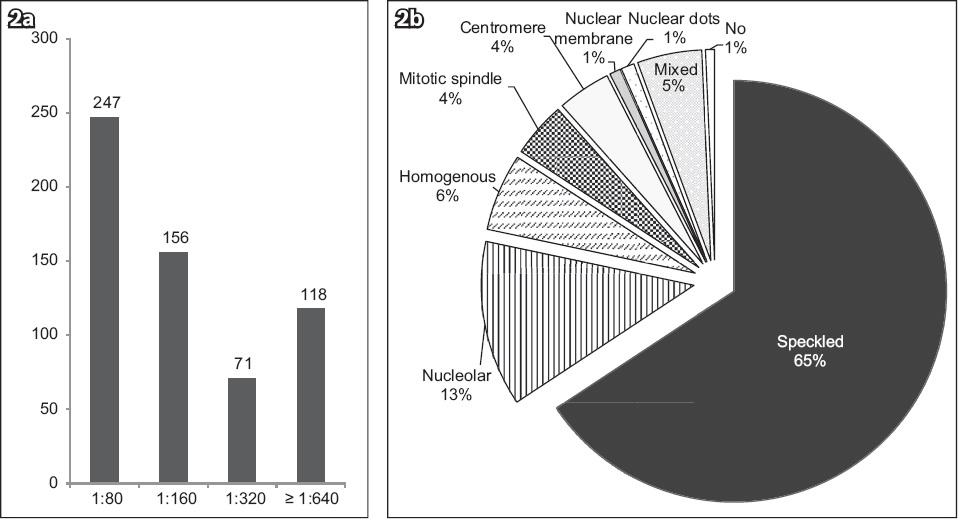
Among the ANA-positive samples, 247 (41.7%) samples were positive at a titre of 1:80, 156 (26.4%) samples at a titre of 1:160, 71 (12.0%) samples at a titre of 1:320 and 118 (19.9%) samples at a titre of over 1:640. The commonest nuclear staining pattern was speckled (n = 388, 65.5%), followed by nucleolar (n = 75, 12.7%) and homogenous (n = 35, 5.9%; Figs.
Fig. 2
Graphs shows (a) ANA titre and (b) nuclear staining pattern in ANA-positive samples. ANA: antinuclear antibody
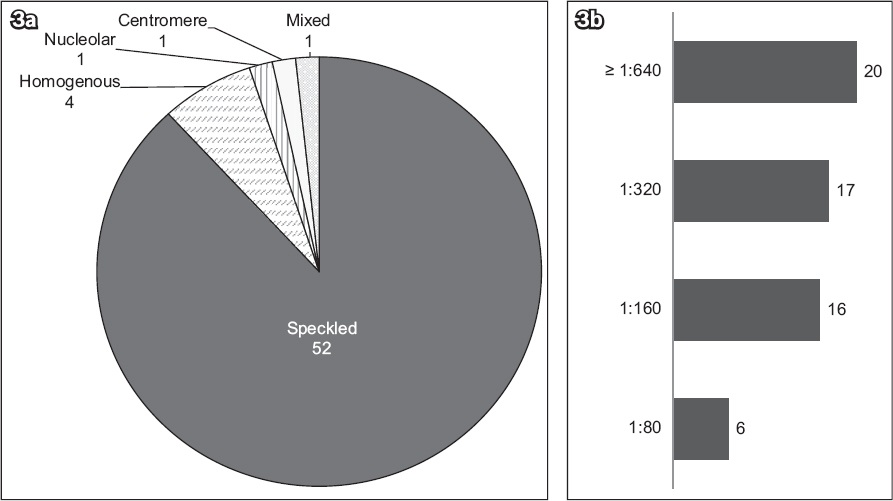
Anti-DFS70 was found to be present in 59 (10.0%) ANA-positive patients. In these 59 anti-DFS70-positive patients, the nuclear staining patterns were described as speckled in 52 (88.1%), homogenous in 4 (6.8%), nucleolar in 1 (1.7%), centromeric in 1 (1.7%) and mixed in 1 (1.7%) patient (
Fig. 3
Graphs show (a) nuclear staining pattern and (b) ANA titre in anti-DFS70-positive samples. ANA: antinuclear antibody; anti-DFS70: 70-kD dense fine nuclear staining protein autoantibody
51 (86.4%) anti-DFS70-positive patients did not have a diagnosis of SARD (
Table I
Serologic profile of anti-DFS70-positive patients with SARD.
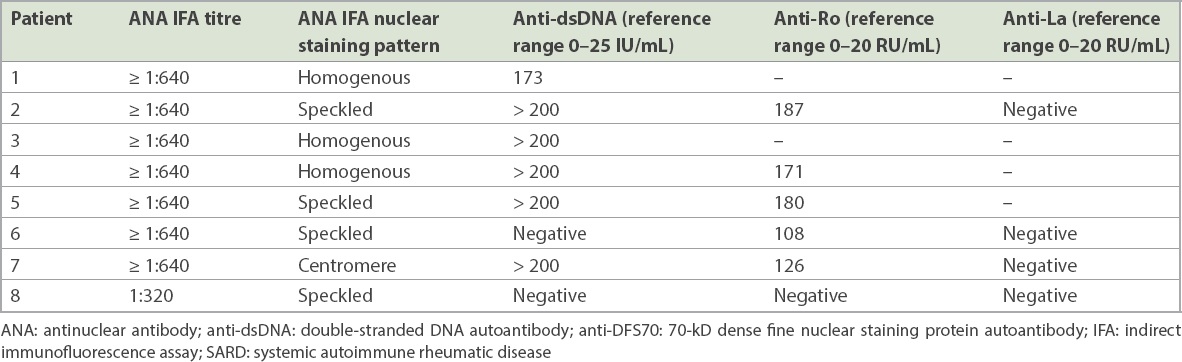
Table II
Clinical profile of anti-DFS70-positive patients with SARD.
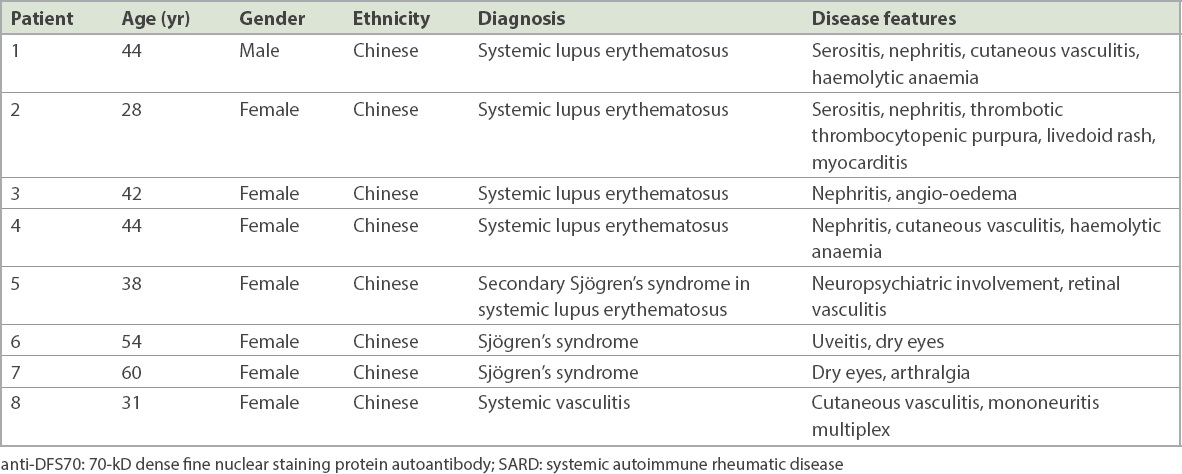
Table III
Cross tabulation of SARD diagnosis with anti-DFS70 findings.
DISCUSSION
The association of anti-DFS70 with disease remains debatable. Reports of associated diseases include SLE, SS, undifferentiated connective disease, Hashimoto’s thyroiditis, Grave’s disease, alopecia areata, multiple sclerosis and Vogt-Koyanagi-Harada syndrome.(12) Anti-DFS70 has also been associated with prostate cancer.(13) A limitation of these earlier studies was that a variety of immunological techniques were used for the detection of autoantibody, including IFA, ELISA and chemiluminescence immunoassay (CLIA). The studies were also performed on small groups of patients, with not more than 334 patients in one disease group based on one review.(6)
Conversely, there have been studies that evaluated the association of anti-DFS70 with healthy individuals, best summarised in a review by Conrad et al.(6) Three key studies have illustrated this association. The first, published by Watanabe et al, evaluated the prevalence of anti-DFS70 via ANA IFA and immunoblot in 597 healthy hospital workers.(14) The prevalence of a positive ANA test in this cohort was 20%, and 54% of the ANA-positive cohort were anti-DFS70 positive, suggesting that anti-DFS70 might have been responsible for a significant proportion of false-positive ANA tests. However, the ANA test is rarely applied to an unselected population; thus, the reported findings may not be clinically meaningful. Dellavance et al sought to address this a year later, in 2005, while evaluating the prevalence of DFS70 nuclear staining pattern on ANA IFA in 13,641 patient samples sent to a general hospital laboratory over a two-year period.(15) The study reported a 37% prevalence of DFS nuclear staining pattern. However, a key limitation of this earlier study was the lack of clinical correlation in the cohort. A sub-analysis of 81 patients with clinical information showed that 61% were diagnosed with non-autoimmune conditions and that the most common autoimmune disease in the remaining 39% was autoimmune thyroiditis. A third key study, published by Mariz et al in 2011, investigated the prevalence of DFS nuclear staining pattern in 118 ANA-positive healthy individuals and 138 ANA-positive patients with SARD.(16) This study found 33.1% of DFS nuclear staining pattern in healthy individuals and none in the SARD group. None of the 41 ANA-positive healthy individuals, followed up over a mean duration of 3.9 years, developed features of SARD at the end of the study. Unfortunately, the prevalence of DFS nuclear staining pattern in these patients was not reported.
The body of evidence over the last two decades has contributed to polarising views over the utility of anti-DFS70 testing. The opposition views the test as one with little discriminatory value in separating health from disease, given its prevalence in a variety of chronic inflammatory diseases, SARDs, cancer and in healthy individuals. The Choosing Wisely Program in the United States(17) is one example of a countermeasure to discrepant ANA testing requests, which aims to educate clinicians on the rational and judicious ordering of serological assays.
On the other hand, proponents of anti-DFS70 testing argue for the adoption of the autoantibody in routine disease-specific autoantibody testing, following a positive ANA test. This is because healthy individuals who are ANA positive tend to be ‘monospecific’ for anti-DFS70. This means that ANA-positive healthy individuals only demonstrate positivity to anti-DFS70, without any coexisting disease-specific autoantibodies, such as anti-dsDNA, anti-Ro and other extractable nuclei antibodies.(12) The addition of anti-DFS70 to a test panel would, thus, have discriminatory value between disease and non-disease states. Additionally, the incorporation of anti-DFS70 into ANA testing algorithms has been argued to be cost-effective, reducing unnecessary specialist clinical referrals and follow-up.(18) Manufacturers of commercial autoantibody test assays appear to subscribe to the proposing view and have adopted the anti-DFS70 test. Numerous commercial anti-DFS70 assays can now be found utilising a variety of immunoassay techniques, including ELISA, CLIA and line immunoassay.
Our study demonstrated a lower prevalence (nearly one-tenth) of anti-DFS70 among our ANA-positive patients than what has been reported in the literature.(14) One possible explanation for this could be the unselected use of the test in a general population in the study by Watanabe et al. In our study, which was performed at an acute tertiary general hospital, clinical specialists would have selected patients with higher pretest probability of disease to perform the ANA test, thus depressing the expected prevalence of the anti-DFS70 in the cohort. 8.6% (n = 51/592) of patients in our ANA-positive cohort had anti-DFS70 and no SARD. Meanwhile, 1.4% (n = 8/592) of ANA-positive, anti-DFS70-positive patients had SARD with other disease-specific autoantibodies. The calculated p-value did not meet statistical significance, suggesting that the presence of anti-DFS70 was not associated with the absence of SARD.
Our study did find similarity with the published literature with regard to the association of anti-DFS70 in patients with SLE and SS.(12,19) The presence of anti-DFS70 in patients with SLE was often found in conjunction with other disease-specific autoantibodies. We were unable to test the hypothesis that a monospecific anti-DFS70 was associated with the absence of SARD, as the Clinical Immunology Laboratory at TTSH does not subject all ANA IFA-positive samples to a full disease-specific autoantibody panel test. Our study was also not designed to evaluate the cost-effectiveness of the anti-DFS70 assay.
In conclusion, the presence of anti-DFS70 was associated with a false-positive ANA test in 8.6% of our study cohort. Anti-DFS70 was not associated with the absence of SARD in our study.


