Abstract
Wünderlich syndrome is a rare entity characterised by spontaneous retroperitoneal haemorrhage with renal origin. We present a case of Wünderlich syndrome secondary to clotting dyscrasia in a 64-year-old woman. The patient experienced a second Wünderlich haemorrhagic event with metachronous pseudoaneurysm formation, which was likely secondary to the large subcapsular haematoma stripping the renal capsule and tearing the cortical arteries. Selective pseudoaneurysm embolisations were successfully performed on both occasions. This clinical entity, its imaging differential diagnoses and management are discussed.
CASE PRESENTATION
A 64-year-old woman with end-stage renal failure secondary to diabetic nephropathy was referred to the emergency department from the dialysis unit following intradialytic hypotension and giddiness. On further questioning, she described a four-day history of dull, non-radiating left flank pain. There was no history of trauma or iatrogenic injury such as renal biopsy.
On examination, the patient had a palpable left flank mass. Admission laboratory serum tests revealed profound anaemia: haemoglobin 4.7 g/dL (normal range [NR] 11.5–15.0 g/dL); haematocrit 14.8% (NR 36.0%–46.0%); and platelet count 320 × 109/L (NR 130–400 × 109/L). Clotting screen was deranged: prothrombin time 11.4 seconds (NR 9.2–11.0 seconds); activated partial thromboplastin time > 190.0 seconds (NR 23.4–36.6 seconds); and international normalised ratio 1.12. Urgent enhanced computed tomography (CT) of the abdomen and subsequent digital subtraction angiography (DSA) were performed (Figs.
Fig. 1
Axial contrast-enhanced CT image of the abdomen taken at the level of the kidneys.
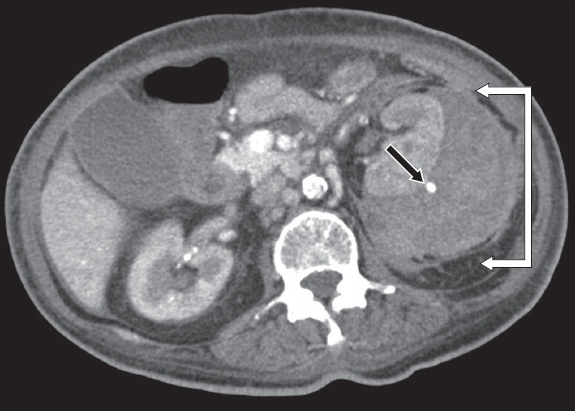
Fig. 2
Digital subtraction angiogram of the left renal artery.
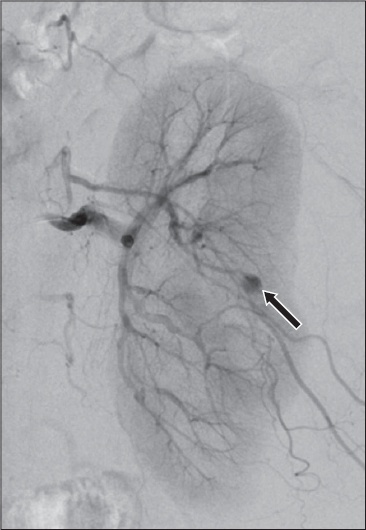
IMAGE INTERPRETATION
Contrast-enhanced CT image of the abdomen shows a large left renal subcapsular haematoma (white arrows) with a 6-mm cortical pseudoaneurysm (black arrow) (
DIAGNOSIS
Wünderlich syndrome and pseudoaneurysm.
CLINICAL COURSE
Following the catheter-directed angiograms, selective pseudoaneurysm embolisation was successfully performed (
Fig. 3
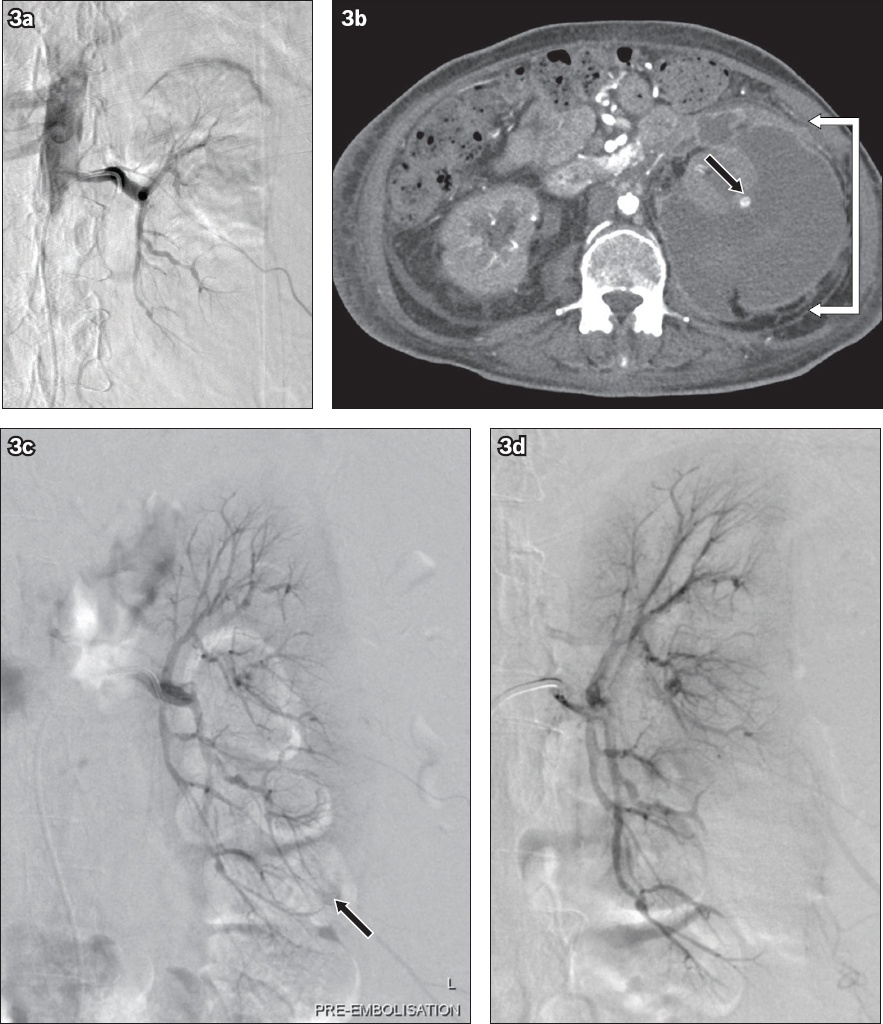
(a) Digital subtraction angiogram (DSA) of the left renal artery after the first embolisation shows no further perfusion of the pseudoaneurysm. (b) Two weeks later, axial contrast-enhanced CT image of the abdomen shows an enlarging subcapsular haematoma with a thick, enhancing wall (white arrows) and a cortical lower pole pseudoaneurysm (black arrow). (c) Pre-embolisation DSA confirms the findings and shows late filling of the cortical lower pole pseudoaneurysm (arrow), while (d) post-embolisation DSA shows no further perfusion of the inferior pole pseudoaneurysm.
Two weeks following the re-admission, the patient had an episode of symptomatic hypotension. Repeat contrast-enhanced CT (
However, two months later, due to worsening ESBL-producing E. coli bacteraemia, the patient underwent open nephrectomy and renal bed washout, which confirmed a large-volume, purulent haematoma. Histological examination of the nephrectomy specimen reported large perinephric and renal capsule abscesses. There was no focal, underlying renal parenchymal mass, or any histological evidence of vasculitis. Postoperatively, recurrent infected haematomas were observed in the renal bed, tracking along the pericolic gutter and into the pouch of Douglas. Following multiple percutaneous drain insertions and prolonged active drain management, the collections finally resolved after three months.
DISCUSSION
Wünderlich syndrome, defined as spontaneous, non-traumatic renal haemorrhage into the subcapsular space, is a rare entity that was first reported in 1856(1) and can only be found in small case series in the literature.(2,3) Patients classically present with the Lenk’s triad of flank pain, palpable mass and hypovolaemic shock. Spontaneous haemorrhage is most commonly attributed to renal tumours such as angiomyolipomas (AMLs;
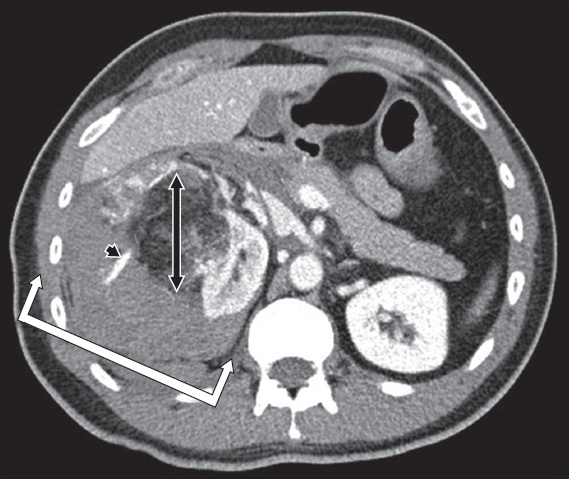
Fig. 4
A 40-year-old man with haemorrhagic rupture of a 6.3-cm right renal angiomyolipoma. Axial contrast-enhanced CT image of the abdomen shows a large right retroperitoneal haematoma (white arrows) secondary to active extravasation (black arrowhead) from a large angiomyolipoma; the latter is diagnosed by its characteristic imaging components of mature adipose tissue, smooth muscle and aberrant vasculature (black arrow).
Imaging plays a vital role to confirm the diagnosis and identify any underlying pathological process that may result in a predisposition to haemorrhage. AML, the most common benign renal tumour associated with spontaneous haemorrhage, especially in lesions that are larger than 4 cm, is a perivascular epithelioid cell tumour consisting of varying proportions of mature adipose tissue, smooth muscle and aberrant vasculature. On CT, it is characteristic but not pathognomonic for AMLs to be visible as macroscopic fat-containing tumours (
RCC is the malignant tumour that is most frequently associated with spontaneous haemorrhage, with features of central necrosis and heterogeneously enhancing solid soft-tissue components. The most common subtype of RCC, clear cell RCC, is usually heterogeneously hypervascular in the arterial phase.(5) It may be difficult to discern ill-defined enhancing soft tissue from adjacent heterogeneously hyperdense haematoma (
Fig. 5
A 78-year-old woman with haemorrhagic rupture of a large RCC. Axial contrast-enhanced CT image of the abdomen shows an anterior mass with mixed soft-tissue enhancement and central necrosis (solid outline) with site of active arterial extravasation (arrow) and secondary mixed attenuating haematoma posteriorly (dashed outline).
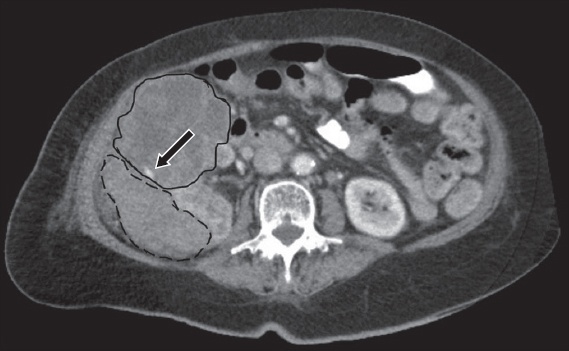
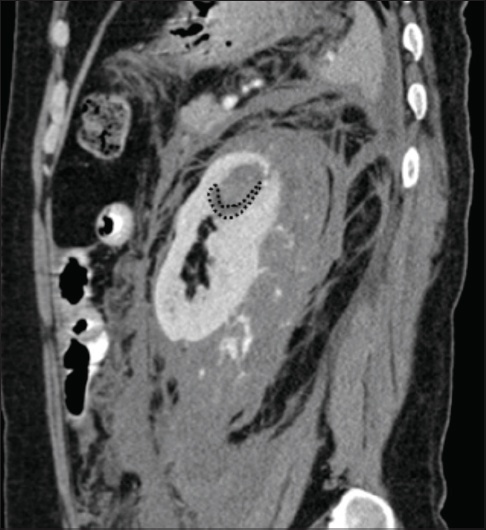
Fig. 6
A 39-year-old man with haemorrhagic rupture of a renal cyst. Sagittal contrast-enhanced CT image of the abdomen shows a faint inferior hypodense rim of the bleeding endophytic renal lesion, consistent with an underlying haemorrhagic cyst (dotted margin).
In the event that there is no discernible aetiology, follow-up CT is recommended to exclude an underlying neoplasm masked by the large-volume haematoma. In our case, the aetiology of the first spontaneous haemorrhage was attributed to the patient’s clotting dyscrasia secondary to heparin use during dialysis, with resulting prolonged prothrombin and activated partial thromboplastin time. The development of a second pseudoaneurysm that was metachronous, anatomically distinct and cortical-based, after a gap of approximately one month, was postulated to have arisen from cortical stripping by the haematoma and subsequent tearing of the cortical arteries. To the best of our knowledge, no previous cases of successfully treated Wünderlich syndrome have resulted in a second delayed haemorrhagic event due to the formation of a new, anatomically distinct pseudoaneurysm. Therefore, despite the confirmed technical success of a transarterial embolisation procedure, it is prudent to maintain a high level of suspicion for a metachronous bleeding site in patients with symptomatic hypotension and recurrent loin pain.
Despite the potential morbidity and mortality of Wünderlich syndrome, it is seldom within the admitting clinician’s list of differential diagnoses.(6) Nevertheless, arterial phase CT is the recommended imaging modality of choice(2) and usually illustrates the diagnosis by revealing a heterogeneously dense subcapsular haematoma with or without a site of bleeding, such as a circular enhancing pseudoaneurysm. However, rarer causes, such as segmental arterial mediolysis, may not be differentiated. Simple episodes of renal subcapsular haemorrhage, in the absence of haemodynamic compromise or pseudoaneurysms, can be managed expectantly. The presence of a pseudoaneurysm, as in this case, requires treatment irrespective of size and symptomatology. Lack of structural support for the damaged arterial wall results in a higher propensity to rupture. Endovascular techniques are increasingly the preferred approach, regardless of aetiology, given their minimally invasive nature, shorter recovery period and ability to preserve renal parenchyma function.(7,8) The precise endovascular treatment, whether it is embolisation with coils, liquid embolics or stent graft exclusion, varies according to the anatomical characteristics of the aneurysm and operator experience. In any case, the primary aim is to occlude or exclude flow to the pseudoaneurysm while maintaining maximal perfusion to the remaining renal parenchyma.(7) Surgery may be required for haemorrhage control or haematoma evacuation in those with haemodynamic instability or uncontrolled sepsis, respectively.(2,6,9) This case of recurrent Wünderlich syndrome was initially managed successfully by transarterial embolisation. However, superadded infection of the large subcapsular haematoma warranted nephrectomy and renal bed washout two months later.
In conclusion, Wünderlich syndrome refers to spontaneous, non-traumatic renal haemorrhage into the subcapsular and perirenal spaces. Despite its severity, it is rarely considered as a clinical diagnosis, but can be confirmed on CT in virtually all cases. Transarterial and open surgical techniques remain complementary in the often challenging management of this condition.
SMJ-58-293.pdf


