Abstract
A 32-year-old man presented to the emergency department with severe right lower limb pain and swelling of three days’ duration. He had multiple prior admissions for recurrent seizures and suicide attempts. Markedly elevated serum creatine kinase levels and urine myoglobinuria were consistent with a diagnosis of rhabdomyolysis. Initial magnetic resonance imaging of the right lower limb revealed diffuse muscle oedema and features of myositis in the gluteal muscles and the adductor, anterior and posterior compartments of the thigh. Follow-up magnetic resonance imaging performed 11 days later showed interval development of areas of myonecrosis and haemorrhage. The causes, clinical presentation and imaging features of rhabdomyolysis are discussed.
CASE PRESENTATION
A 32-year-old Chinese man with a history of recurrent seizures, depression and multiple suicide attempts by drug overdose presented to the emergency department with severe right lower limb pain and swelling of three days’ duration. He suffered an episode of seizure upon admission. On examination, the right thigh and calf were significantly swollen and tender. There was no significant neurovascular compromise of the right lower limb. Laboratory markers revealed significant elevation of serum creatine kinase (CK) level at > 4,100 U/L and urine myoglobinuria at 1,409 µg/L. Serum creatinine was also elevated at 329 µmol/L. Mild hyperkalaemia was noted at 5.5 mmol/L. A review of the patient’s medications revealed that he had been on multiple prescription drugs, notably the selective serotonin receptor inhibitor (SSRI) escitalopram 15 mg every night for depression. Magnetic resonance (MR) imaging of the pelvis and right thigh was performed at the time of admission (Figs.
Fig. 1
Coronal and axial turbo inversion recovery magnitude MR imaging sequences of the pelvis and right thigh (a & b) at the time of admission and (c & d) 11 days following admission when the patient’s symptoms worsened.
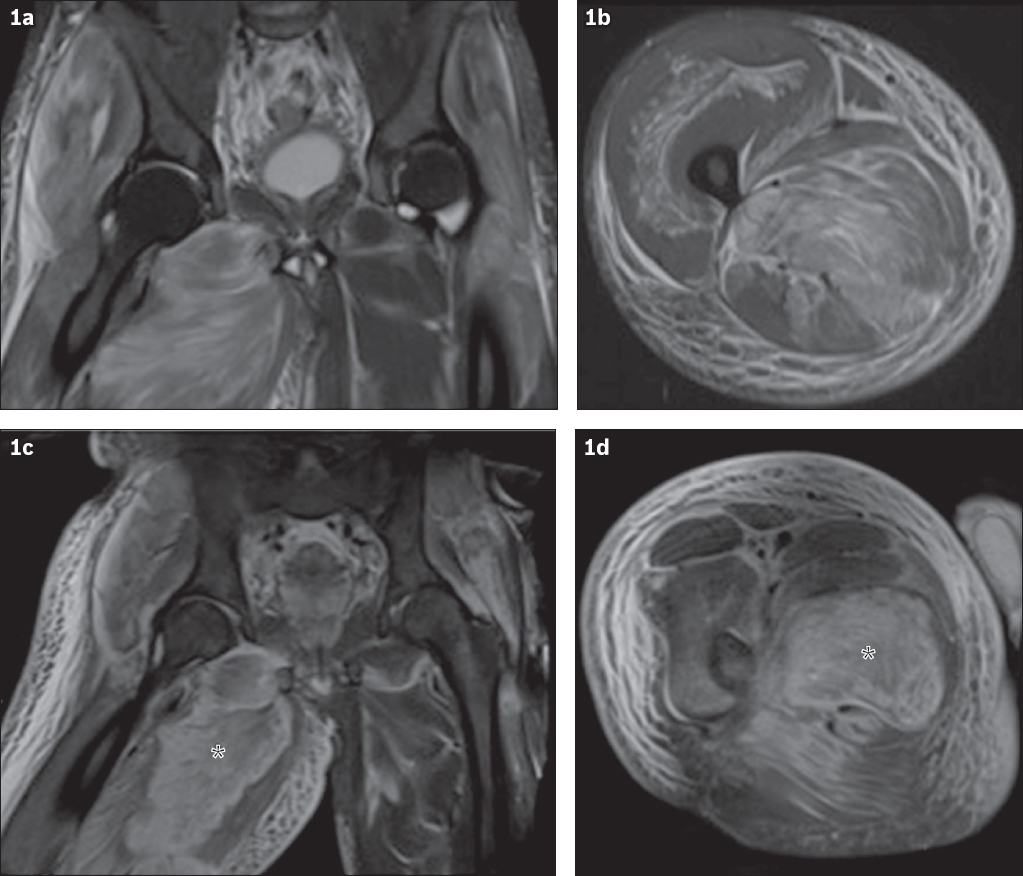
IMAGE INTERPRETATION
At the time of admission, coronal and axial turbo inversion recovery magnitude (TIRM) sequences show diffuse hyperintensity and swelling in the bilateral gluteal and right adductor compartments (
DIAGNOSIS
Severe rhabdomyolysis complicated by myonecrosis.
CLINICAL COURSE
The patient was aggressively rehydrated intravenously, but his renal function and serum CK levels failed to improve after two days. He was then transferred to the medical intensive care unit and commenced on continuous veno-venous haemofiltration (CVVH), with subsequent improvements in his renal function and serum CK levels. His right lower limb neurovascular status remained intact, which excluded the possibility of an evolving compartment syndrome. No attributable autoimmune or viral cause of the patient’s rhabdomyolysis was elucidated. He was scheduled for further evaluation with electromyography and muscle biopsy, but these were not performed, as the patient requested for hospital discharge against medical advice.
DISCUSSION
Acute rhabdomyolysis should be recognised early and promptly treated, as it has potentially deleterious consequences. The condition occurs as a result of striated muscle breakdown, which causes the release of various intracellular contents, including CK, aldolase, myoglobin, potassium, carbonic anhydrase and lactate dehydrogenase.(1-3) Although the spectrum of clinical presentation is varied, the symptoms of cramp-like pain, swelling and loss of function of the affected muscle group (more commonly the large muscle groups of the thigh, pelvis or paraspinal region) usually predominate. There may be accompanying nonspecific symptoms such as malaise, lethargy and gastrointestinal discomfort. Tea-coloured urine is a classical description in textbooks and is due to myoglobinuria.(2,3)
Laboratory markers greatly aid in the diagnosis of rhabdomyolysis. A markedly elevated serum CK level, in particular the CK-MM subtype that is found in striated muscle, is highly sensitive for muscle damage. The presence of urine myoglobinuria is an important additional finding for confirmation, although the absence of myoglobinuria does not exclude rhabdomyolysis. Muscle biopsy, while confirmatory, is usually not required.(1-3)
The causes of rhabdomyolysis include substance abuse (alcohol and illicit drugs), trauma, excessive muscular activity, prescription drug use (e.g. salicylates, fibrates, statins, anaesthetic and antidepressive agents), seizures and infection.(2-4) In the present case, our patient was known to be on the SSRI escitalopram, for treatment of depression. SSRIs have an established association with rhabdomyolysis.(4) A variety of mechanisms have been postulated to explain drug-induced rhabdomyolysis. Toxins may induce vasospasm, cause involuntary muscle contraction, affect muscular metabolism and interfere with the metabolism of adenosine triphosphate.(4)
More importantly, the attending clinician should be aware of the potential complications of rhabdomyolysis, which include electrolyte abnormalities, hypovolaemia, acute renal failure, disseminated intravascular coagulation, metabolic acidosis, compartment syndrome and liver dysfunction.(1-3) In a retrospective evaluation of 475 hospitalised patients with rhabdomyolysis, Melli et al found that acute renal failure secondary to myoglobinuria was present in nearly half of the patients (46%).(3) Likewise, our patient also developed acute renal failure, and his renal function improved only after he was treated with CVVH.
MR imaging, computed tomography (CT) and ultrasonography may be helpful in the radiological evaluation of rhabdomyolysis. Muscle abnormalities may be broadly classified into three patterns: muscle oedema, fatty infiltration and mass lesion.(5,6) MR imaging has superior soft-tissue contrast resolution and the highest sensitivity among the different imaging modalities available for the detection of rhabdomyolysis.(7,8) Diffuse T2-weighted hyperintensity with corresponding T1-weighted hypointensity in the affected muscle groups, the most common finding described on MR imaging, represents diffuse oedema. Inversion recovery sequences, such as TIRM and short-tau inversion recovery (STIR), are helpful in accentuating contrast between abnormal and normal muscle, as well as adjacent fat, due to inherent fat suppression. Diffuse oedema in rhabdomyolysis may progress to myonecrosis and appear ‘mass-like’.(5)
Two distinct types of MR imaging findings may be seen in rhabdomyolysis, as proposed by Lu et al in 2007.(7) In Type 1, which occurs in cases of overexertion, the involved muscles usually appear homogeneously isointense to hyperintense on T1-weighted imaging, and homogeneously hyperintense on T2-weighted and STIR sequences. Type 2 is encountered in cases of direct trauma, vascular occlusion, carbon monoxide poisoning, as well as alcohol and illicit drug abuse. Muscles affected by Type 2 rhabdomyolysis usually present with homogeneous or heterogeneous hyperintense signal on T1-weighted imaging and heterogeneously hyperintense signal on T2-weighted sequences. This is in contrast to Type 1 MR imaging findings, in which the signal change of the affected muscles on both T1- and T2-weighted images is usually homogeneous. A characteristic ‘stippled’ sign may also be present, causing a dot-like or streaky linear appearance within a region of rim enhancement on contrast-enhanced MR imaging. The appearance of Type 2 findings has been postulated to be due to remnant muscle fibres or inflammatory vasculature within necrotic muscle, representing areas of myonecrosis.(7)
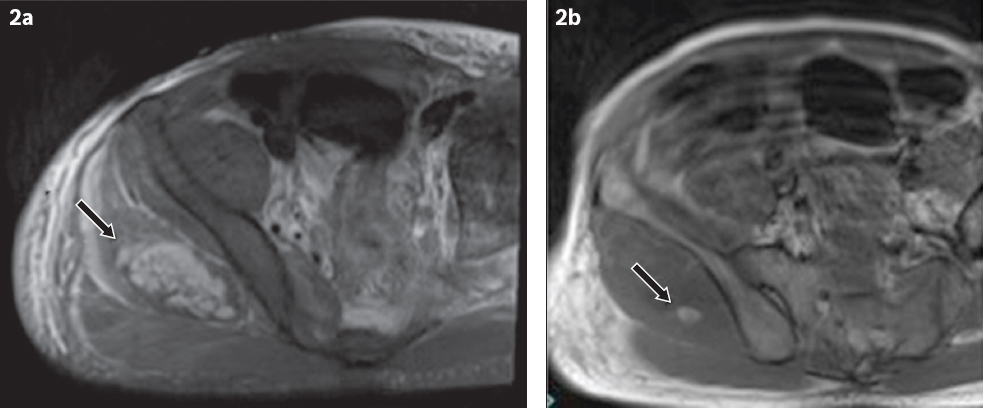
Areas of intramuscular haemorrhage, which appear hyperintense on T1-weighted images and hypointense on gradient-recalled echo images, have also been described in cases of rhabdomyolysis.(8,9) Similarly, follow-up MR imaging of our patient showed an area of myonecrosis in the right gluteus medius (
Fig. 2
(a) Axial turbo inversion recovery magnitude MR image shows focal myonecrosis in the gluteus medius (arrow). (b) Axial T1-weighted MR image shows focal hyperintensity within the region of myonecrosis, likely representing haemorrhage (arrow).
On CT imaging, involved muscle groups usually demonstrate low attenuation due to underlying oedema, with occasional rim-enhancement on contrast-enhanced imaging. However, these are nonspecific imaging findings that can also be seen in pyomyositis and infiltrative intramuscular neoplasms.(7,10,11) On ultrasonography, affected muscle groups generally appear hypoechoic, with effacement of the normal striated architecture of the muscle fibres, although these findings are also nonspecific.(11,12)
In the absence of relevant clinical history and laboratory markers, radiological findings of rhabdomyolysis may be difficult to distinguish from those of inflammatory myopathy, muscle infection, necrotising fasciitis or other conditions associated with myonecrosis such as diabetes mellitus or sickle cell crisis.(5,6,9,13-18) Nonetheless, certain clinical clues and radiological patterns may help to guide imaging interpretation. For example, inflammatory myopathies secondary to autoimmune causes, such as polymyositis or dermatomyositis, usually present with slowly progressive onset of weakness and classically involve the symmetrical proximal muscles, which appear hyperintense on fluid-sensitive sequences (e.g. STIR) and fat-saturated contrast-enhanced T1-weighted sequences.(13)
Muscle infection (i.e. pyomyositis) is occasionally seen in immunocompromised patients and usually involves the large muscle groups of the thigh and pelvis.(14,15) It appears on MR imaging as hyperintense focal lesions with significant perilesional oedema, occasionally progressing to abscess formation (
Fig. 3
A 36-year-old Malay man with poorly controlled diabetes mellitus and pyomyositis of the left calf. (a) Axial turbo inversion recovery magnitude and (b) sagittal proton density-weighted MR images show a hyperintense focus (arrows) within the left soleus with surrounding muscle oedema. (c) Axial T1 fat-saturated post-contrast MR image shows a rim-enhancing collection in the soleus (arrow) with enhancement of the surrounding soleus and gastrocnemius.
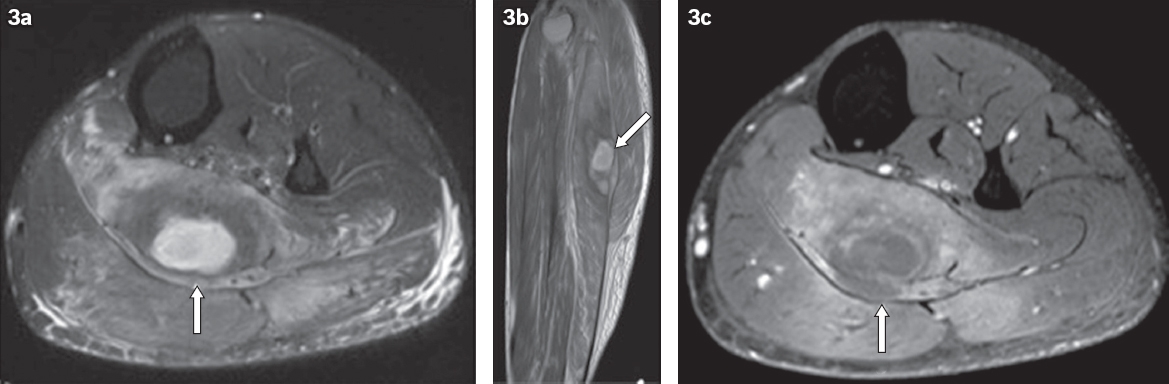
Fig. 4
A 31-year-old intravenous drug user with necrotising fasciitis of the right thigh. (a) Axial turbo inversion recovery magnitude MR image shows a thickened deep fascia and intermuscular fluid in the medial and posterior compartments of the right thigh (arrows). (b) Axial T1-weighted MR image shows mildly raised signal in the adductor magnus, probably due to haemorrhage (arrow). (c) Axial T1 fat-saturated post-contrast MR image shows hypo-enhancing muscle in the medial and posterior compartments of the thigh, suspicious for necrosis (arrow).
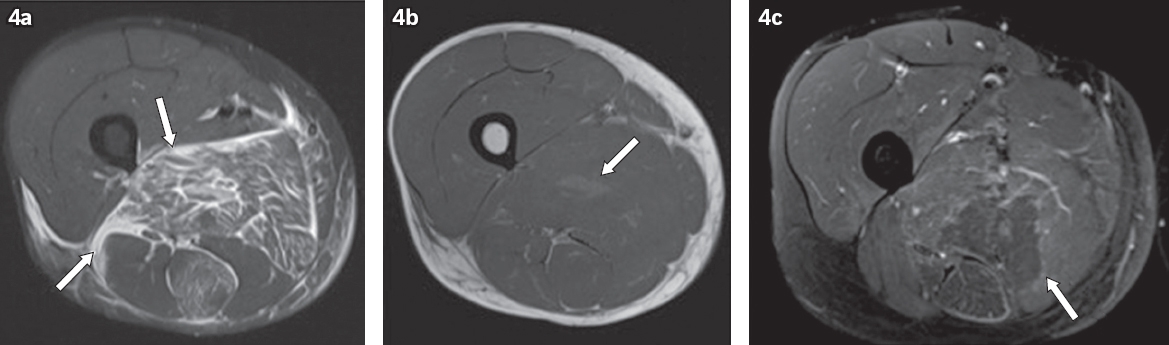
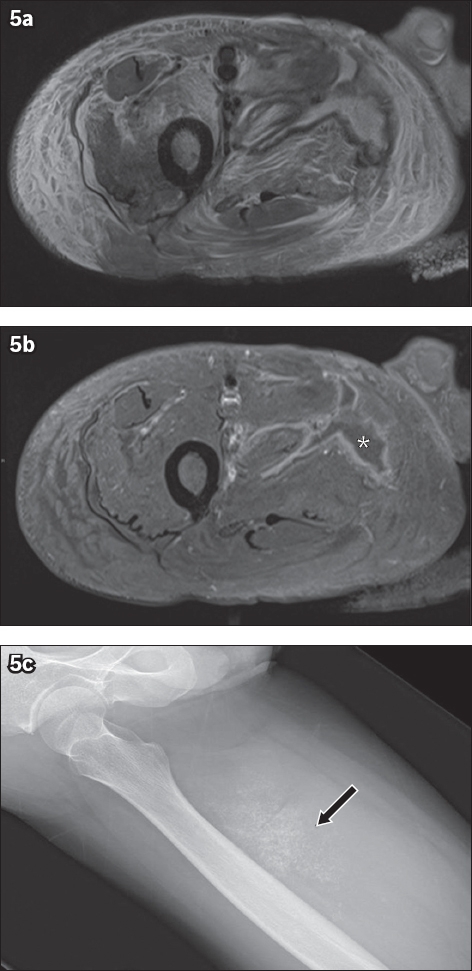
Fig. 5
A 57-year-old Caucasian woman with diabetic myonecrosis of the right thigh. (a) Axial turbo inversion recovery magnitude and (b) axial T1 fat-saturated post-contrast MR images show marked oedema of the anterior and medial compartment muscles, associated with an irregular non-enhancing area of myonecrosis (asterisk in 5b) and subcutaneous oedema. (c) Follow-up radiograph of the thigh shows dystrophic calcifications at the site of myonecrosis (arrow).
In summary, this article illustrates the progression of imaging findings in a case of severe rhabdomyolysis that was complicated by myonecrosis. The imaging diagnosis of this condition should be made in tandem with corroborative clinical and laboratory findings, as there is a potential overlap of imaging features with other infective and inflammatory conditions. MR imaging, however, remains clinically useful for assessing the overall extent and severity of rhabdomyolysis, as well as in monitoring for progression or resolution of muscular signal abnormalities.
SMJ-58-472.pdf


