Abstract
A 26-year-old woman presented with a slow-growing right breast lump. Excision biopsy of the lump showed invasive ductal carcinoma with adjacent ductal carcinoma in situ (DCIS). Preoperative imaging was performed to assess the extent of disease. Magnetic resonance (MR) imaging of the breasts showed an area of clustered ring enhancement deep to the biopsy site, which was representative of residual DCIS. DCIS is a common noninvasive malignancy that manifests as a primary breast tumour or in association with other lesions. The radiological features of DCIS are discussed herein, with special attention to the clustered ring enhancement pattern on MR imaging.
CASE PRESENTATION
A 26-year-old woman presented with a six-month history of a right breast lump that was gradually increasing in size. The patient reported occasional tenderness of the lump but no overlying skin changes, nipple discharge or fever. She was nulliparous and had no previous personal or family history of breast cancer. She had started taking an oral contraceptive six months prior to the presentation of the lump, but otherwise had no significant medical or surgical history. On physical examination, a few small lumps were palpable in the upper outer quadrant of the right breast near the axillary tail. There was no nipple discharge or overlying skin changes. No enlarged axillary or supraclavicular lymph nodes could be felt bilaterally. Examination of the left breast was normal.
Initial breast ultrasonography showed an indeterminate group of masses in the upper outer quadrant of the right breast, which corresponded with the findings of the physical examination. The patient was offered a percutaneous core needle biopsy of the lump for histological diagnosis, but she opted for a surgical excision biopsy instead. Histology revealed breast malignancy. Post-surgery breast imaging workup with mammography, ultrasonography and magnetic resonance (MR) imaging (Figs.
Fig. 1
Mammograms of both breasts in (a) craniocaudal and (b) mediolateral oblique views.
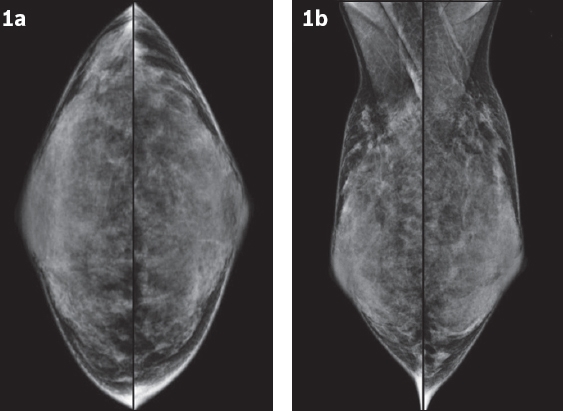
Fig. 2
Selected US images of the right breast.
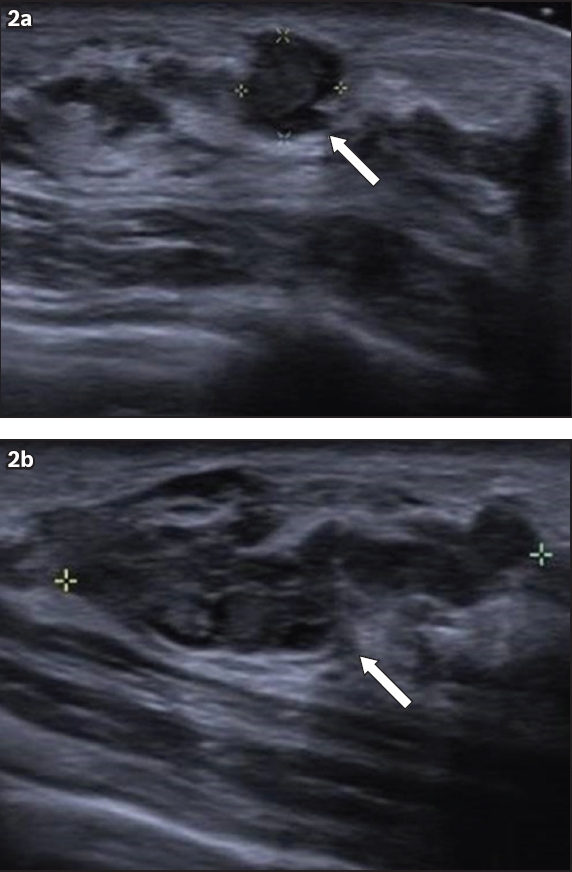
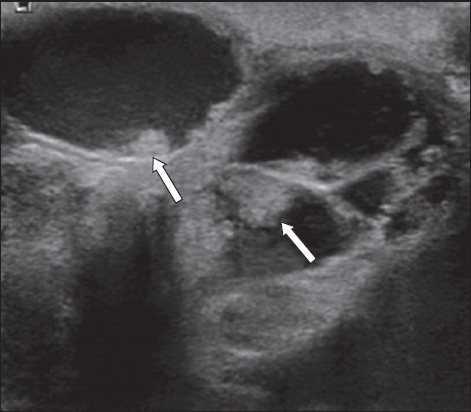
Fig. 3
Selected (a) sagittal and (b) axial contrast-enhanced T1-W MR images of the right breast.
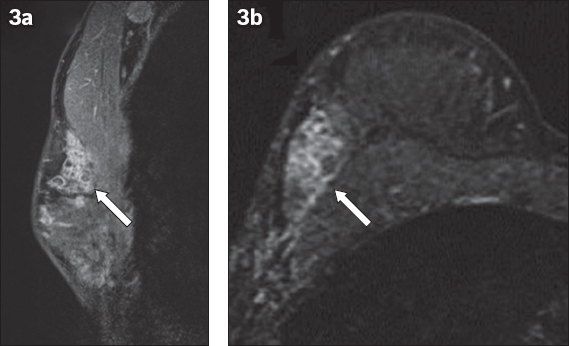
IMAGE INTERPRETATION
The postoperative mammograms (
DIAGNOSIS
Ductal carcinoma in situ (DCIS).
CLINICAL COURSE
Mammography and MR imaging were performed after the excision biopsy to assess the extent of disease, as part of planning for definitive breast surgery. The primary abnormal area of enhancement was fairly extensive on MR imaging, involving most of the upper outer quadrant. There were other separate, smaller segmental areas of heterogeneous non-mass enhancement in the periareolar regions of the ipsilateral breast, which were also suspicious for DCIS (not shown in images). The patient was offered biopsy of these other separate areas of enhancement if she was keen on breast conservation, but she eventually opted for mastectomy. A right skin-sparing mastectomy with sentinel lymph node biopsy was performed, followed by breast reconstruction with a pedicled latissimus dorsi flap. The mastectomy pathological specimen measured 11 cm × 8 cm × 3 cm, with residual areas of low to intermediate nuclear grade papillary DCIS, as well as intraductal papillomatosis in the right upper outer quadrant. These showed strong and diffuse nuclear staining for oestrogen receptors and more variable reactivity for progesterone receptors. There was also non-contiguous low-grade DCIS in the periareolar regions, which probably correlated with the other separate, non-mass enhancing areas on MR imaging. Sentinel lymph node biopsy was negative for metastasis, and postoperative recovery was uneventful. The patient received genetic counselling, and her BRCA1 and BRCA2 mutations were negative. She underwent adjuvant chemotherapy with docetaxel and cyclophosphamide. Left ovarian cryopreservation was performed prior to the initiation of chemotherapy.
DISCUSSION
DCIS is a pre-invasive cancer that consists of malignant epithelial cells in the terminal ductal lobular unit of the breast, with no invasion across the basement membrane surrounding the duct. In the majority of cases, DCIS is asymptomatic. With the current breast screening protocols, it is mostly detected via screening mammography. DCIS can also occasionally present clinically as a breast lump or pathological nipple discharge, or in the form of Paget’s disease. In these symptomatic cases, clinical correlation with breast imaging is essential. Ultrasonography is the most important breast imaging modality due to its high sensitivity, ease of use and ability to provide direct, real-time imaging correlation with the palpable abnormality. Diagnostic mammography, which is also frequently performed concurrently, not only improves diagnostic imaging sensitivity, but also improves specificity. It also helps in assessing the extent of the disease and screens for other separate suspicious areas in the ipsilateral and contralateral breasts.
MR imaging is recognised as the most sensitive imaging modality in the detection of breast malignancy, including DCIS.(1,2) However, its role in the initial workup of breast symptoms is limited due to its high cost and moderate level of specificity. Another reason for its limited role is the ability of ultrasonography and mammography to resolve most of these initial diagnostic problems. MR imaging is useful in pre- and perioperative assessments of newly diagnosed breast cancer, especially in patients who are suitable for and keen on breast-conserving surgery. In addition to screening for high-risk breast cancer, the universally accepted American College of Radiology recommendations also indicate the use of breast MR imaging for the following reasons: assess the extent of disease before definitive breast surgery; screen for additional and separate malignant disease in the ipsilateral and contralateral breasts; monitor neoadjuvant chemotherapy response; distinguish between recurrence and post-surgical scarring; and identify occult primary breast tumours.(3) It is also used for diagnostic problem-solving on the rare occasion when findings of clinical examination, mammography and ultrasonography are inconclusive or discordant.
Mammography
DCIS is commonly detected as an area of suspicious microcalcifications on mammography. Microcalcifications are a common finding on mammography and most are benign. However, microcalcifications that come in clusters of different size, shape and density (pleomorphic morphology) or appear linear-branching with a ductal or segmental distribution strongly suggest DCIS. Approximately 10% of these cases may also be associated with a mass or area of architectural distortion.(4) While pure DCIS can present as a mass, one should also consider the presence of an invasive component when DCIS with a mass is detected. Up to 22% of DCIS cases can present without microcalcifications and up to 44% can be mammographically occult.(5-7) In such cases, DCIS may only be detectable on ultrasonography or MR imaging, as illustrated in the present case.
Ultrasonography
DCIS is uncommonly detected on ultrasonography, because it does not frequently form sonographically detectable masses. DCIS-associated microcalcifications, if present, are better visualised and characterised on mammography. On ultrasonography, DCIS can appear as ill-defined, irregular masses, or as mass-like areas with altered heterogeneous echotexture, which may or may not be palpable. It can also appear as abnormal intraductal masses (
Fig. 4
US image shows ill-defined, hypoechoic ductal carcinoma in situ masses (arrows) associated with several echogenic foci, which represent abnormal microcalcifications. The branching masses appear tubular in configuration and extend toward the nipple, in keeping with a ductal configuration.
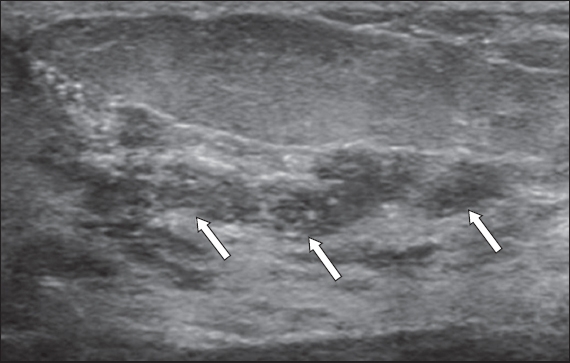
Fig. 5
US image shows a complex, septated solid-cystic mass in an encysted papillary ductal carcinoma in situ. The solid component is irregular (arrows) and the cystic components are likely to be dilated blocked ducts. The echoes within the cystic components are related to debris, necrotic cells and haemorrhagic products.
The ultrasonographic appearance of masses with a branching and tubular configuration suggests ductal abnormalities. They may show extension toward the nipple and may be associated with cystic ductal components. The main differential diagnoses for such ductal masses on ultrasonography are papillomas (
Fig. 6
US image shows a duct leading to the nipple filled with an elongated, well-defined intraductal solid nodule (arrow) that contains a few foci of microcalcifications. Surgical excision following imaging-guided hookwire localisation revealed papillomatosis.
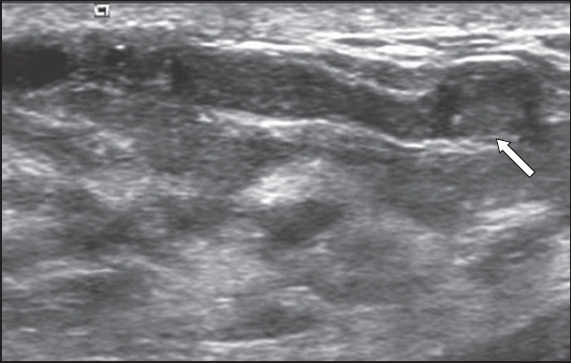
Fig. 7
US image shows ductal ectasia with internal echoes (arrow). On real-time US imaging, the internal echoes are mobile and are related to either ductal debris or inspissated material, rather than an intraductal solid nodule. It is likely a benign finding, although it should be further correlated clinically for any pathological nipple discharge.
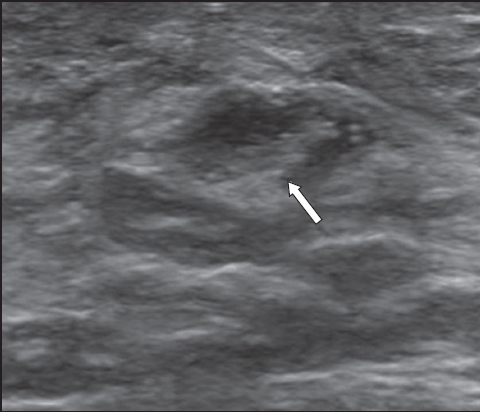
Fig. 8
US image shows extensive tubular masses over the left upper outer quadrant with increased vascularity (arrow), corresponding with a clinically palpable lesion. Histology from the mastectomy specimen revealed extensive intermediate nuclear-grade ductal carcinoma in situ.
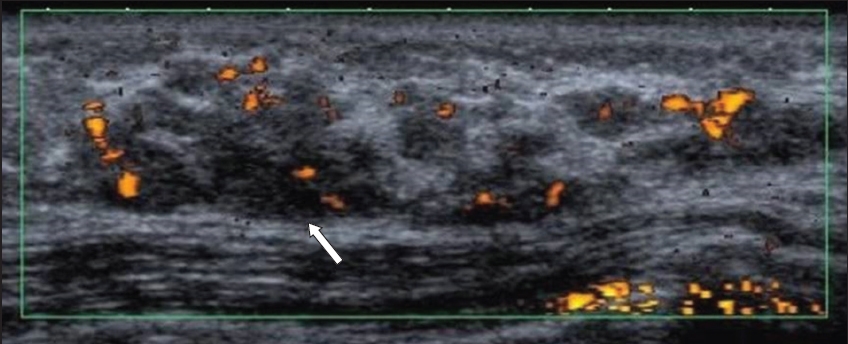
Fig. 9
US image shows a dilated duct at the periareolar 9 o’clock position containing a large, irregular intraductal mass (arrow). The mass was found to be invasive ductal carcinoma.
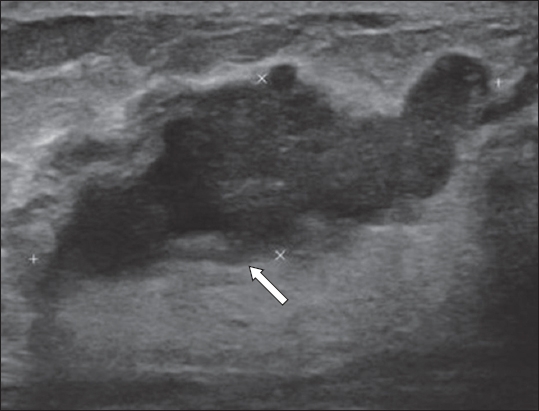
Fig. 10
US image shows a diffuse area containing tubular- and nodular-type masses (arrow) that correspond with a palpable lesion. US-guided vacuum-assisted biopsy revealed nodular sclerosing adenosis.
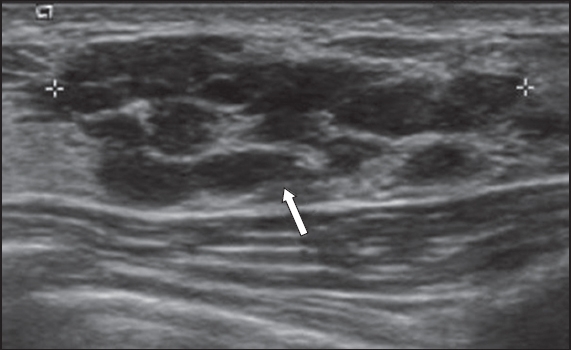
Fig. 11
US images (a & b) show a subareolar tubular lesion communicating with a nodule (arrows). (b) Vascular flow is noted at the periphery of the nodule. US-guided vacuum-assisted biopsy revealed granulation mastitis.
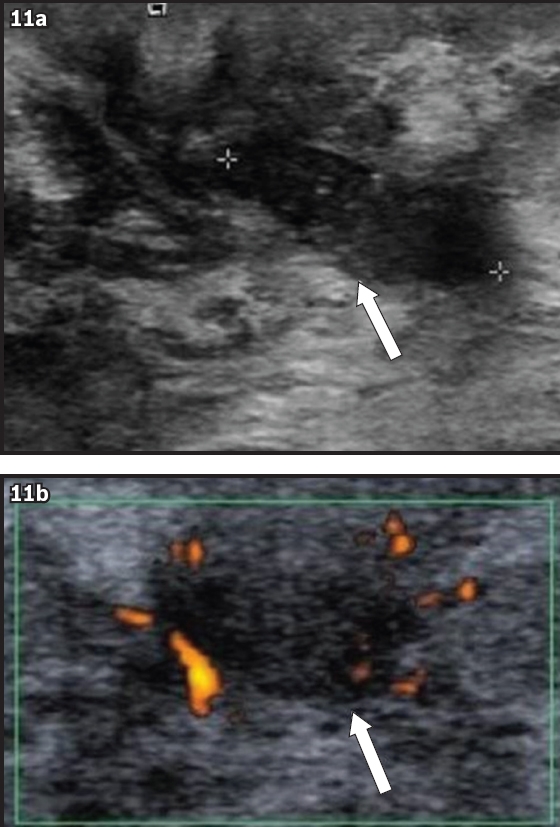
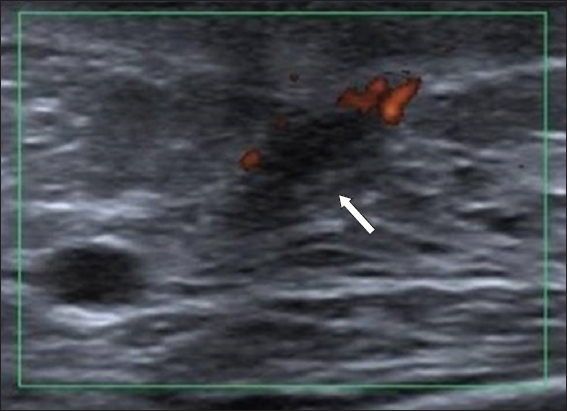
Fig. 12
US image shows a subareolar distended duct with internal echoes (arrow), corresponding with a low-density nodule seen on mammogram (not shown). There is increased vascularity along the borders of the duct. US-guided vacuum-assisted biopsy revealed benign ductal ectasia with chronic periductal mastitis.
While the significance of ductal ectasia is nonspecific, it is usually benign and commonly found together with periductal inflammation. However, malignancy can also be associated with ductal ectasia; any co-existing intraductal mass should be biopsied to assess for DCIS if it has immobile internal echoes, irregularly thickened ductal wall or intraluminal microcalcifications with suspicious morphology. Asymptomatic ductal ectasia without any suspicious imaging features can usually be left alone. However, in cases where the patient has pathological nipple discharge, surgical microdochectomy should be considered despite the benign-appearing imaging findings. Breast abscess and periductal mastitis should be treated accordingly, and do not need to be biopsied unless they do not resolve with treatment or do not demonstrate the typical clinical findings of infection. For other intraductal masses, biopsy is generally recommended, especially since benign papillomas can be associated with malignancy. This rule is applied in our institution, although for asymptomatic, small (< 1 cm) intraductal nodules without overt suspicious morphological features that are incidentally identified on imaging, we offer patients the option of clinical and imaging surveillance as an alternative to biopsy.
Biopsy options for ductal masses detected via ultrasonography include ultrasonography-guided percutaneous core biopsy and surgical excision biopsy. Percutaneous options include the use of a spring-loaded core needle (commonly known as a Tru-cut biopsy needle) and vacuum-assisted biopsy needle. In contrast to open surgical biopsy, percutaneous biopsy is convenient and minimally invasive, resulting in the avoidance of the need for general anaesthesia as well as significantly less post-procedural scarring, if at all. Percutaneous biopsy also helps in the planning of definitive surgery when malignancy is detected. There is an increasing preference for vacuum-assisted biopsy for ductal masses because a vacuum-powered biopsy needle is able to rotate and draw multiple large core tissue samples into the needle. This allows for complete or near-complete excision of the lesion after repeated sampling, enabling a more accurate histological outcome. The less costly Tru-cut biopsy, on the other hand, captures smaller breast tissue in the sampling notch of the thin biopsy needle after a rapid forward throw from a spring-loaded, handheld biopsy device. With the thinner and smaller core samples, only part of the mass is sampled. It is also difficult to ascertain if the biopsy needle has traversed through small masses for accurate tissue sampling. This is a problem because ductal masses can be very small. In addition, benign papillomas and breast malignancies are the main differentials of ductal masses, and they can coexist, giving rise to sampling errors when the masses are not adequately sampled. A few studies have shown that Tru-cut core needle biopsies of papillary lesions have a small upgrade rate to papillary DCIS in subsequent breast excision surgeries.(13,14) As such, a pathologist is more likely to ask for a complete specimen excision when given Tru-cut samples. Although a vacuum-assisted biopsy is more costly than a Tru-cut core needle biopsy, it has the advantage of obtaining a more definitive histological sampling, which obviates the need for further surgery if complete or near-complete excision of a benign mass is achieved. It can also be therapeutic for removing palpable benign breast lumps and stopping non-clinically important nipple discharge.
MR imaging
MR imaging is considered to be the most sensitive modality available for identifying DCIS, particularly high-grade DCIS. It is also a superior imaging technique for assessing the extent of disease, especially in the preoperative setting, which is useful in the assessment and planning of breast conservation surgery.(4,15,16) DCIS usually manifests as non-mass enhancement (NME)(17) and occasionally, as enhancing masses. The internal enhancement pattern that is commonly associated with DCIS is the clumped pattern,(15) while segmental and ductal types of distribution are also suspicious for DCIS. MR imaging kinetics is not useful in the assessment of NME, and the evaluation of morphology takes precedence in such lesions. Clustered ring enhancement is a recently recognised pattern of NME that is strongly associated with DCIS. The sign is now an accepted descriptor in the fifth edition of the BI-RADS (Breast Imaging Reporting and Data System) MR imaging lexicon. It is postulated that this sign represents periductal enhancement and contrast pooling in the ductal walls.(18) Tozaki et al first described this abnormal pattern of MR imaging enhancement in 2006, and it has been found to have a 77%–100% positive predictive value for malignancy, mostly related to DCIS.(18-20)
The clustered ring enhancement sign has also been found occasionally in invasive breast cancers and benign pathologies such as atypical ductal hyperplasia, papillomas and complex sclerosing lesions, which by themselves are associated with an elevated risk of breast cancer.(15,16) Indeed, DCIS can co-exist with these elevated-risk benign lesions, which makes adequate sampling essential. Yuen et al recommends that when the clustered ring enhancement sign is present on MR images, a biopsy with a thicker core needle (such as a vacuum-assisted biopsy needle) should be performed, as DCIS may only be present focally. In the event that the histological outcome from MR-guided percutaneous biopsy is benign, surgical resection should be considered to exclude malignancy.(21)
Differentials of the clustered ring enhancement sign on MR imaging may include masses that show ring enhancement. Invasive breast cancers can manifest as multiple ring-enhancing lesions (
Fig. 13
Fat-suppressed, contrast-enhanced T1-W MR image shows several irregular masses with rim enhancement in the left breast (arrows), which was later histologically proven to be multicentric invasive breast carcinoma.
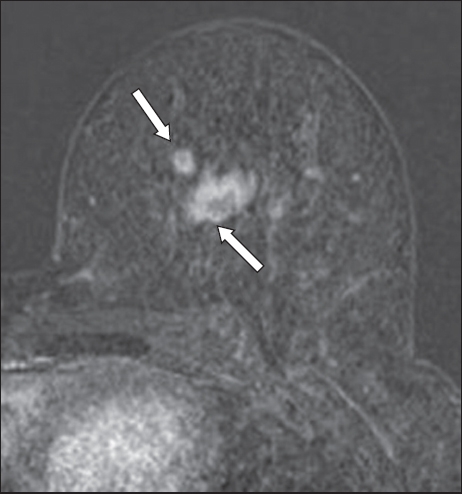
Fig. 14
(a) Fat-suppressed, contrast-enhanced T1-W MR image shows a small cyst with wall enhancement in the left breast (arrows in a & b). (b) T2-W spectral adiabatic inversion recovery image shows a bright cyst, in keeping with fluid signal. This represents an inflamed cyst.
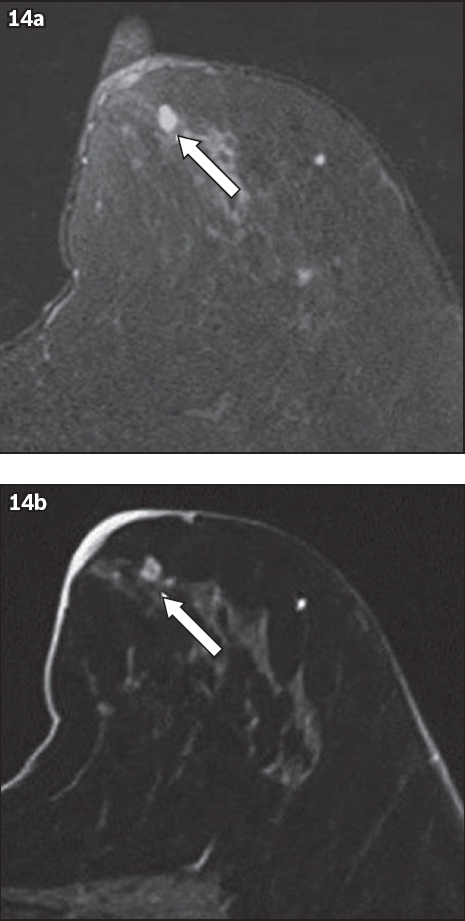
Fig. 15
Fat-suppressed, contrast-enhanced T1-W MR image shows a post-surgical seroma (arrow) after an excision biopsy for a previous lesion in the right breast, which histology later revealed to be invasive lobular carcinoma. The cavity shows a thin rim of enhancement with no residual-enhancing mass at the cavity margins.
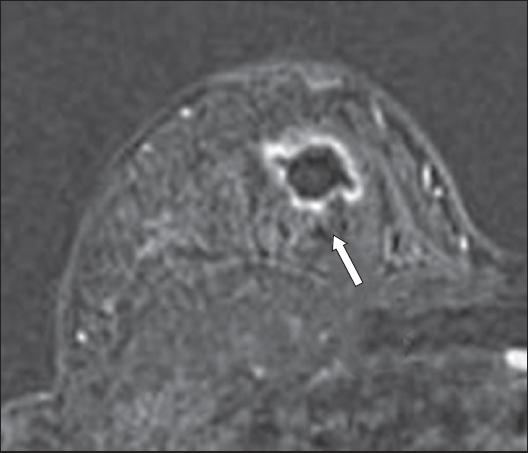
Fig. 16
Fat-suppressed, contrast-enhanced T1-W MR image shows a deep, irregular left breast abscess with prominent enhancement of its walls (arrow).
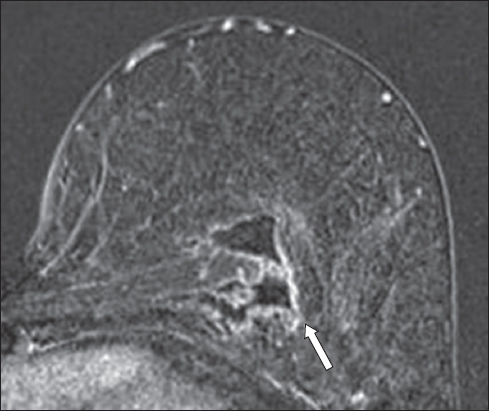
Fig. 17
A patient with prior right skin-sparing mastectomy and axillary clearance for right breast invasive ductal carcinoma, with a single metastatic right axillary lymph node. Fat-suppressed, contrast-enhanced T1-W MR image shows variable peripheral rim enhancement and central fat-containing signal (arrow). This proved to be fat necrosis on subsequent fine-needle aspiration.
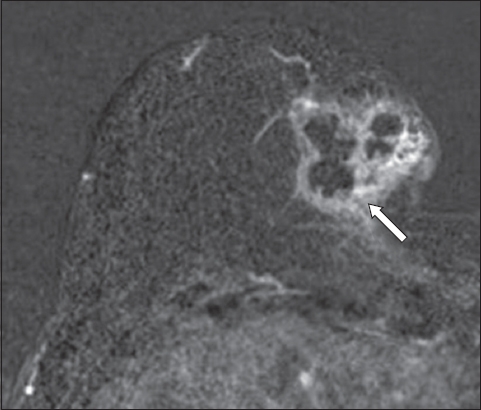
Fig. 18
Fat-suppressed, contrast-enhanced T1-W MR image shows fairly homogeneous areas of enhancement at the periphery of the left breast, displaying a ‘picture-frame’ appearance. This represents normal breast tissue enhancement.
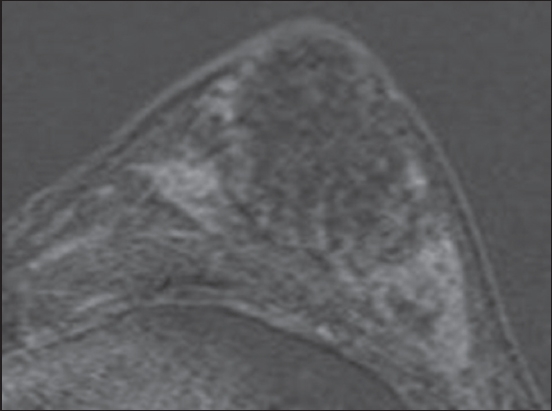
In conclusion, the findings of ductal type abnormality on ultrasonography and the clustered ring enhancement sign on MR imaging should be recognised and carefully evaluated with triple assessment for the presence of DCIS.
SMJ-58-592.pdf


