Abstract
A 60-year-old woman was travelling on a plane with her spouse when she suddenly developed acute-onset disorientation to time and space. According to her husband, she repetitively questioned her whereabouts and complained of a minor headache. Upon landing, she was immediately brought to the emergency room and subsequent magnetic resonance imaging was performed, which showed multiple punctate 2–3 mm regions of diffusion-weighted imaging hyperintensity in the medial aspects of both temporal lobes. The conglomeration of clinical history and radiological findings was most suggestive of transient global amnesia. This article discussed the background, proposed mechanisms, diagnosis, radiological characteristics and management of transient global amnesia.
CASE PRESENTATION
A 60-year-old woman with no significant past medical history was travelling on a plane with her spouse when she suddenly developed acute onset of disorientation to time and space. According to her husband, she repeatedly asked where she was and complained of a minor headache. Throughout the episode of disorientation, the patient remained conscious and was cognisant of her identity. The patient’s memories prior to boarding the plane were fully preserved. No focal abnormality was noted on neurological examination. Magnetic resonance (MR) imaging was performed (
Fig. 1
DW imaging sequences at the level of (a) the cerebral peduncles and (b) lower mid-brain.
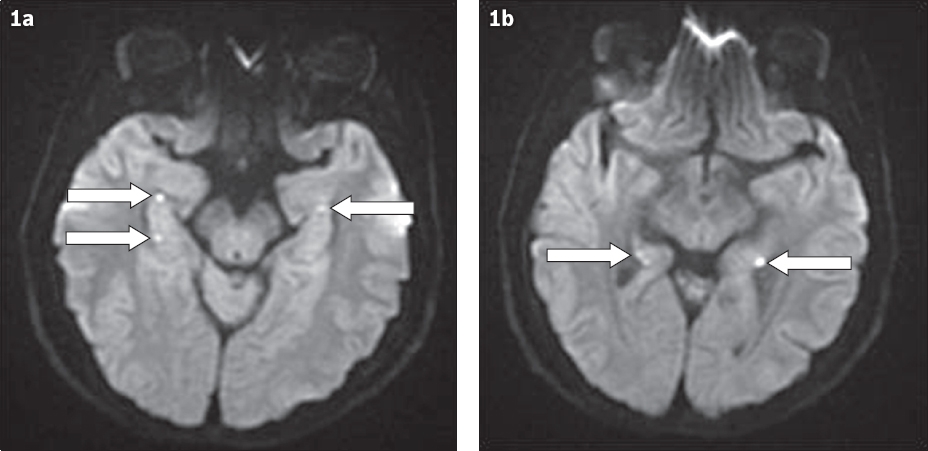
IMAGE INTERPRETATION
Axial MR sequences through the level of the temporal lobes show several punctate 2–3 mm regions of diffusion-weighted (DW) imaging hyperintensity in the region of the lateral hippocampi (arrows in Figs.
DIAGNOSIS
Transient global amnesia (TGA).
CLINICAL COURSE
The patient was admitted to a neurology ward for observation. Her disorientation gradually and spontaneously resolved over the course of 12 hours without intervention. The patient was subsequently discharged with outpatient follow-up. A short amnestic gap remained and she was unable to recall the events that occurred during the acute episode.
DISCUSSION
TGA manifests as a sudden and striking onset of anterograde amnesia that spontaneously resolves within 24 hours. Age is the only known risk factor,(1) as it predominantly affects middle-aged people between the fifth and seventh decades of life. TGA has an incidence of up to 32 per 100,000 among those aged over 50, with an equal distribution between both genders and all racial groups.(2)
Although it was first documented in 1958, the pathophysiology behind TGA remains poorly understood.(3) A number of mechanisms have been proposed, including arterial ischaemia, venous congestion, migraine and epilepsy.(4) However, convincing evidence to support these theories remains scant. With respect to the most popular theory of arterial ischaemia, a number of case-control studies have been unable to demonstrate a relationship between TGA and an increase in cerebrovascular risk factors (e.g. blood pressure and serum cholesterol) or other anatomical cardiac abnormalities.(5) Furthermore, follow-up studies comparing TGA patients to transient ischaemic attack (TIA) patients have actually shown a significantly lower incidence of future stroke in the TGA group.(6)
Although the pathophysiology of TGA is unclear, the clinical presentation is quite characteristic and relatively specific. Currently, the diagnosis is made solely based on clinical criteria (
Radiology can be particularly useful in confirming the presence of TGA in cases where the clinical criteria for TGA are not fully satisfied. The presence of solitary or multifocal 1–3 mm punctate regions of restricted diffusion within the unilateral or bilateral hippocampi is very suggestive of TGA.(4,8,9) According to Sedlaczek et al, based on a retrospective study of 31 cases, MR imaging may be positive in only 6% of cases in the first few hours after the initial attack. This percentage rises to 84% if scans are performed 48 hours after the initial symptoms.(8) These detection rates were based on DW imaging slice thicknesses of 5 mm and b-values of 1000 s/mm2 and 2000 s/mm2.(8) Further refinement of DW imaging parameters has improved the detection rate for TGA lesions to up to 88% using a slice thickness of 2 mm, a b-value of 2000 s/mm2 and repeat imaging at 72 hours.(10) Therefore, if TGA is suspected, optimising DW imaging parameters and performing delayed imaging 48–72 hours after presentation will allow for the highest possible sensitivity.
MR signal abnormality affecting the hippocampi has a limited radiological differential diagnosis. A recent paper published by Förster et al(9) described how to differentiate patterns of diffusion restriction on DW imaging sequences from the more common processes affecting the hippocampus, such as infarction, TGA, seizure and limbic encephalitis. For example, the classic imaging appearance of TGA is of discrete, punctate 1–3 mm DW imaging lesions that are usually located towards the lateral aspect of the hippocampus (
Fig. 2
Comparison of DW patterns of hyperintensity within the hippocampus. (a) MR image of transient global amnesia shows a punctate focus (arrow) of signal abnormality in the left hippocampus. (b) MR image of posterior cerebral artery (PCA) infarct shows a larger region of signal abnormality (arrow) in the right hippocampus with associated patchy DW signal in the right occipital lobe (PCA distribution). (c–e) MR images of seizure-related change, with corresponding (d) T2-W and (e) FLAIR images, show increased signal abnormality (arrow in 2c) and swelling in the right hippocampus (arrow in 2d) that reversed upon subsequent imaging (arrow in 2e). (f–h) MR images of limbic encephalitis with corresponding (g) T2-W and (h) FLAIR images which show signal abnormality and swelling in both hippocampi (arrows).
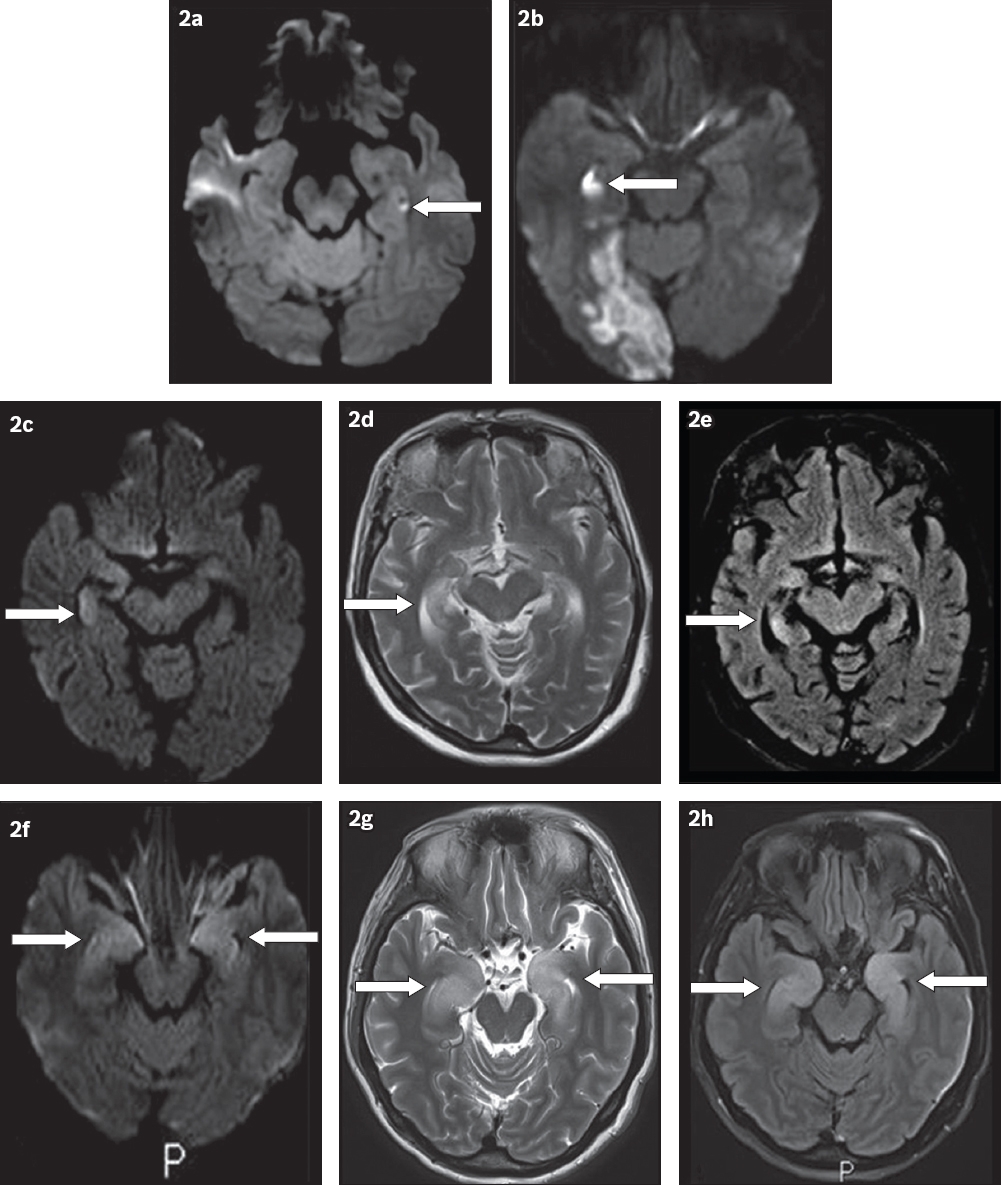
The clinical differential diagnosis for the presentation of isolated short-term retrograde amnesia with the inability to form new memories includes TGA, vascular entities such as TIA/stroke, transient epileptic amnesia and, less commonly, a toxic or metabolic disturbance. A full neurological examination and history can usually confirm the diagnosis in most cases. Clinically, epileptic amnesia will typically be of a shorter duration, and may present with automatisms such as lip smacking or abnormal electroencephalography (EEG) findings.(12) Toxic or metabolic states such as hypoglycaemia usually have features that are consistent with global cognitive dysfunction, such as a delirious state.
In the case of a vascular process such as TIA, an embolism is more likely to shower across multiple small arterioles(9) to produce a more diffuse clinical picture involving more than just retrograde and anterograde amnesia. Nevertheless, if there is still diagnostic uncertainty after a full neurological exam, blood tests for electrolyte balance, glucose levels and complete blood count, toxicology screen, EEG and neuroimaging with MR imaging are recommended.
Once diagnosed, the prognosis for TGA is largely favourable. Episodes commonly resolve within six hours, but may last up to 24 hours before resolving spontaneously. Patients can be left with a permanent amnestic gap corresponding to the episode of anterograde amnesia. Besides a brief amnestic gap, patients will have no long-term neurological deficits, and the risk of reoccurrence is relatively low, in approximately 5% of patients.(13) Management should include watchful waiting, outpatient follow-up and repeat MR imaging if clinically indicated.(1)
SMJ-59-355.pdf



