Abstract
A 44-year-old man presented with breathlessness and episodes of palpitations for the last one year. The imaging diagnosis of cardiac sarcoidosis was made based on chest radiography and cardiac magnetic resonance (MR) imaging findings, and was further confirmed by biopsy. Cardiac sarcoidosis is an uncommon entity, yet is potentially fatal with nonspecific clinical manifestations, including sudden cardiac death. Hence, it is important to diagnose and treat this entity at an early stage to improve morbidity and mortality. Cardiac MR imaging plays a pivotal role in facilitating diagnosis and monitoring therapeutic response. We describe the MR imaging features of cardiac sarcoidosis and discuss imaging features of other cardiomyopathies that may mimic cardiac sarcoidosis.
CASE PRESENTATION
A 44-year-old man presented to the emergency department with breathlessness and a history of intermittent episodes of palpitation for the past one year, which had increased in frequency to almost every day (2–3 times per day) over the last two weeks. Each episode lasted for about 10–15 minutes. The first electrocardiogram (ECG) showed features of right bundle branch block, followed by subsequent ventricular tachycardia. The patient was started on verapamil and amiodarone, and was given 150 joules of electrical cardioversion. His troponin T and creatine kinase levels were high at 175.4 (0–14.0) pg/mL and 666 (24–200) U/L, respectively. Echocardiogram showed concentric left ventricular (LV) hypertrophy with hypokinetic wall motion and LV systolic dysfunction. What do the chest radiograph (
Fig. 1
Frontal chest radiograph of the patient taken at the time of presentation.
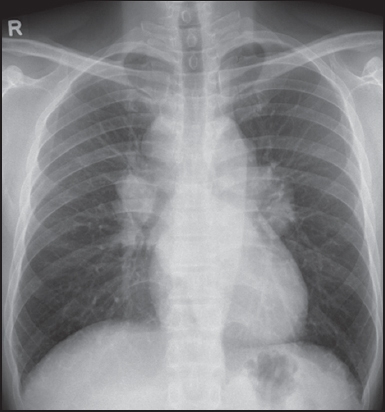
Fig. 2
Short-axis (a) T2-W, (b) early contrast-enhanced and (c) late gadolinium enhancement (LGE) MR images.
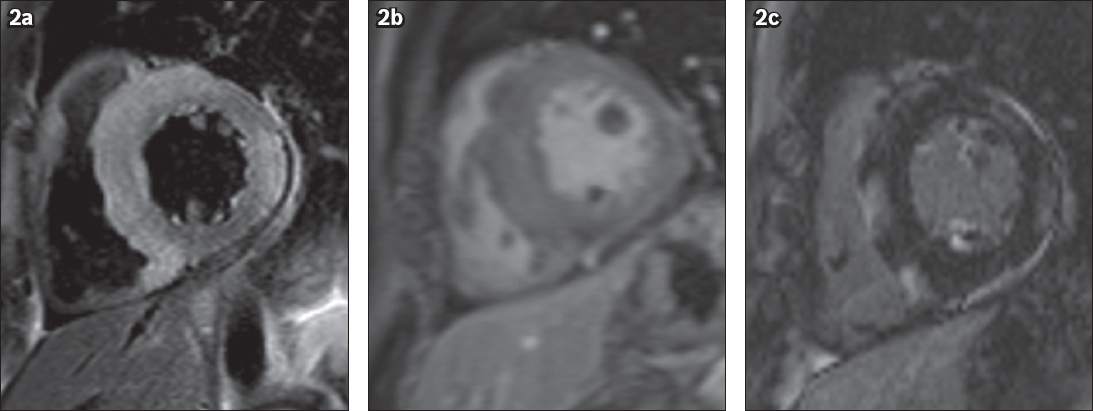
IMAGE INTERPRETATION
The frontal chest radiograph (
CMR imaging confirmed mediastinal and bilateral hilar lymphadenopathy. In addition, it shows extensive confluent areas of increased T2 signal intensity (corresponding to oedema) in the septum, anterior septal segment and inferior septal segment that extended into the adjoining right ventricle wall and in the lateral wall of the LV (
Fig. 3
(a) Axial diffusion weighted (b-1000) and (b) apparent diffusion coefficient map MR images show patchy areas of restricted diffusion in the septum and the left ventricular wall.
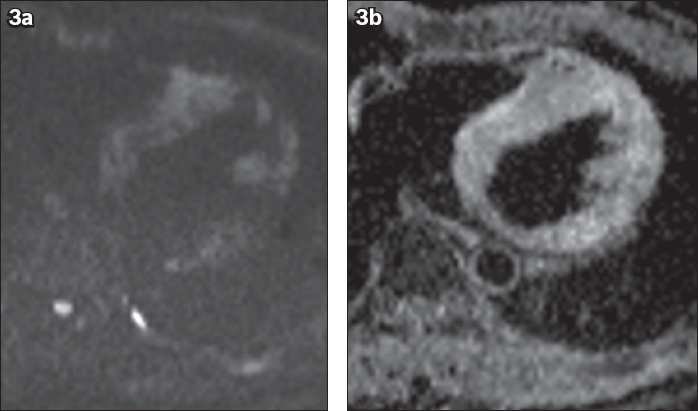
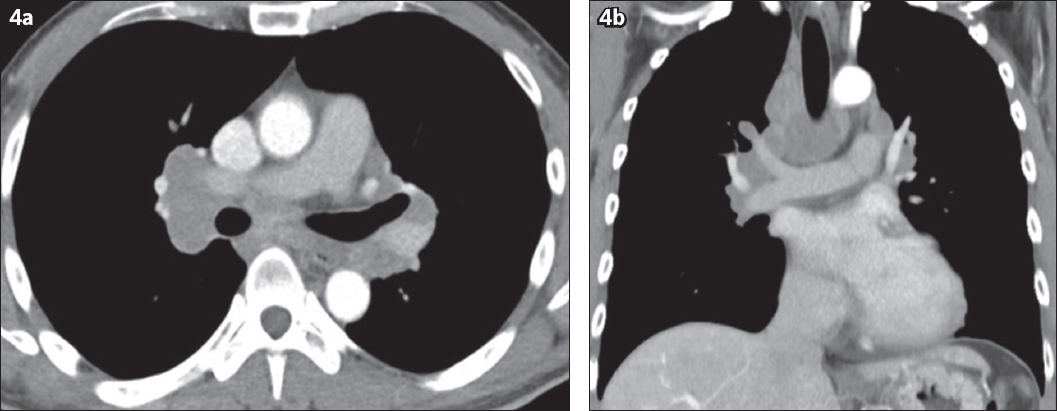
Contrast-enhanced computed tomography of the thorax was performed, which also shows bilateral symmetrical hilar lymphadenopathy with extensive bilateral paratracheal, aortopulmonary window and left para-aortic lymphadenopathy (
Fig. 4
(a) Axial and (b) coronal reformatted contrast-enhanced CT images of the thorax show bilateral symmetrical hilar and mediastinal lymphadenopathy.
DIAGNOSIS
Acute phase cardiac sarcoidosis (CS).
CLINICAL COURSE
The patient was referred to the rheumatology department and started on intravenous hydrocortisone for five days and intravenous methylprednisolone for three days. Following video mediastinoscopy and mediastinal lymph node biopsy, histopathology confirmed the diagnosis of sarcoidosis. An automated implantable cardioverter-defibrillator was inserted a few days later, and the patient was discharged and started on oral amiodarone 200 mg, bisoprolol 2.5 mg and prednisolone 60 mg. A month later, the patient presented with acute renal failure due to nephritis and blurring of vision due to posterior uveitis, both secondary to sarcoidosis. No further imaging was performed in the last three months (from the time of first presentation).
DISCUSSION
Sarcoidosis is characterised by the formation of non-caseating granulomas affecting multiple organs.(1) Sarcoidosis typically manifests as bilateral hilar lymphadenopathy and pulmonary nodules. Other organs commonly involved include the skin, eye and central nervous system.(2) Cardiac involvement, characterised by infiltrative cardiomyopathy, is clinically rare and diagnosed in only 5% of patients, even though around 20%–50% patients may show non-caseating granulomas within the myocardium on autopsy.(2,3) Patients with CS may be completely asymptomatic or may present with non-specific symptoms such as chest pain, palpitations, dyspnoea and syncope.(2) It can be life-threatening, causing fatal ventricular or supraventricular tachyarrhythmia, LV dysfunction, conduction disturbances and sudden death.(1) Approximately 12%–65% patients with CS can succumb to sudden death from dysrhythmias.(2)
CS histologically progresses through three stages: oedema, granulomatous inflammation and fibrosis leading to scarring.(3) Myocardial biopsy is highly specific and the gold standard method for diagnosing cardiac sarcoidosis. However, it is invasive and may have a false-negative result due to the patchy pattern of the disease.(1,4)
Imaging diagnosis of CS is challenging and requires good clinical, radiological and pathological collaboration. CMR imaging, with its high spatial and soft tissue contrast, has the capability to detect the acute inflammatory phase of the disease and the chronic phase, which includes fibrosis and scarring.(5) In the acute inflammatory phase, CMR imaging shows focal wall thickening and regional wall motion abnormalities, while the granulomas demonstrate patchy nodular areas of increased T2 signal intensity within the myocardium, with early enhancement and LGE due to oedema associated with inflammation.(5,6) CS commonly involves the septum, particularly the basal septum and LV free wall, whereas right ventricular involvement is quite rare.(6,7) With septal involvement, contiguous involvement of the right ventricular insertion point is often seen, as in our case.(3) CS may affect any portion of the myocardium, the commonest being transmural involvement. Focal non-transmural lesions commonly affect the subepicardial or mid-myocardial portion and less commonly, the subendocardial portion.(2,8) In severe cases, CMR imaging may show diffuse myocardial thickening secondary to massive granulomatous infiltration, resulting in significant contraction abnormalities and cardiac failure.(6) The myocardial thickening and LGE usually resolve after steroid treatment, while increased T2 signal within the myocardium may persist.(9)
In the chronic phase, CMR imaging usually shows focal or diffuse thinning of the myocardial wall with LGE, without high T2 signal or early gadolinium enhancement, indicative of myocardial damage, fibrosis and scarring.(5,10) The LGE occurs due to increased volume of contrast material in the extracellular space secondary to underlying myocardial fibrosis.(2) LGE is considered to be the strongest hallmark of CS and a marker of adverse events such as ventricular arrhythmias and sudden death.(2,5) Cine CMR images are useful in demonstrating regional wall motion and contraction abnormalities associated with advanced disease.(2) CMR imaging was shown to have a sensitivity and specificity of 100% and 78%, respectively, for diagnosing sarcoidosis.(1,11) LGE sequence is a very important CMR imaging protocol for identifying both active and chronic phases of CS, and plays a key role in both diagnostic and prognostic assessments and in evaluating response to steroid treatment.(1,2) Patel et al showed that the rates of adverse events and cardiac death were nine and 11.5 times higher, respectively, in patients with LGE on CMR imaging in CS than in patients who did not exhibit LGE.(12) After the initiation of treatment, patients with lower LGE show good response compared to those with severe LGE who either show no response or have worse outcomes.(9) Associated findings that may be identified on CMR imaging, such as right ventricular hypertrophy and dilatation, are probably due to pulmonary hypertension secondary to underlying lung disease.(6)
Other noninvasive imaging modalities, such as echocardiography and nuclear scintigraphy (thallium-201 and gallium-67), may be used to diagnose CS but have limited success. Fluorine-18 fluorodeoxyglucose positron emission tomography (i.e. 18F-FDG PET) may be useful for diagnosing CS, but is limited by cost and availability.(1)
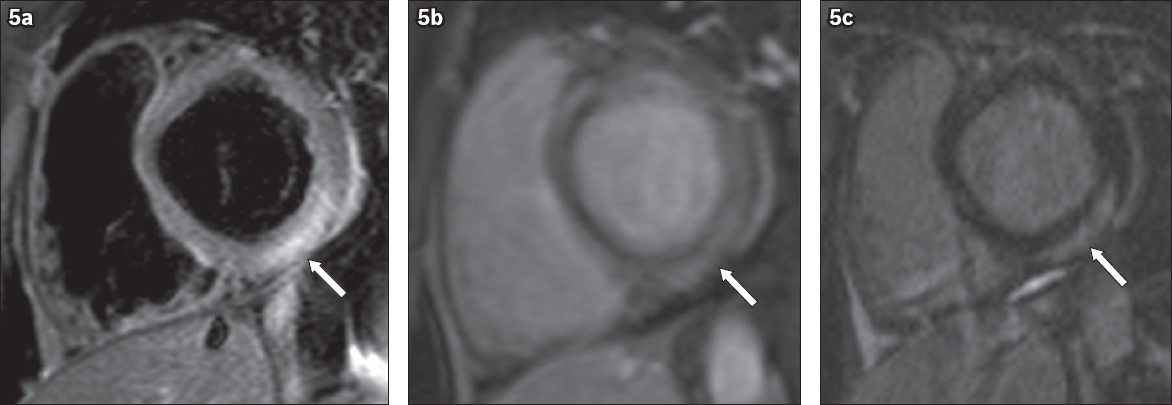
Other cardiomyopathies that may demonstrate overlapping imaging features with CS include myocarditis, hypertrophic cardiomyopathy, ischaemic cardiomyopathy and amyloidosis.(2) These conditions, however, show different patterns of myocardial involvement. Myocarditis shows increased T2 signal in the myocardium with early enhancement and LGE, particularly in the epicardial distribution. Unlike CS, myocarditis frequently involves the lateral free wall of the LV rather than the interventricular septum (
Fig. 5
A 39-year-old man with acute myocarditis. (a) Short-axis T2-W MR image shows high signal intensity in the lateral and inferior segments (arrow), indicative of oedema. (b) Short-axis early contrast-enhanced and (c) LGE MR images show subepicardial early enhancement and LGE in the inferior and inferolateral segments (arrow in b & c).
In CS, diffuse granulomatous infiltration may result in extensive myocardial thickening, as in our case, and this can morphologically mimic hypertrophic cardiomyopathy. The diagnostic criterion for hypertrophic cardiomyopathy on CMR imaging is LV wall thickness ≥ 15 mm at end-diastole and characteristically involves the interventricular septum, most obviously in the anteroseptal myocardium.(2,14) In hypertrophic cardiomyopathy, LGE commonly involves the septum (particularly the basal portion) and the junction between the right ventricular free wall and interventricular septum, and is more likely to be mid-myocardial.(2) In contrast to CS, hypertrophic cardiomyopathy does not commonly show myocardial oedema, which is seen as increased T2 signal (
Fig. 6
A 50-year-old man with asymmetrical (septal) hypertrophic cardiomyopathy. (a) Short-axis T2-W MR image shows asymmetric septal wall hypertrophy at the anteroseptal wall, with the maximal thickness (arrow) measured as 18 mm at end-diastole. (b) Short-axis LGE MR image shows mid-myocardial LGE in the anteroseptal wall (arrow).
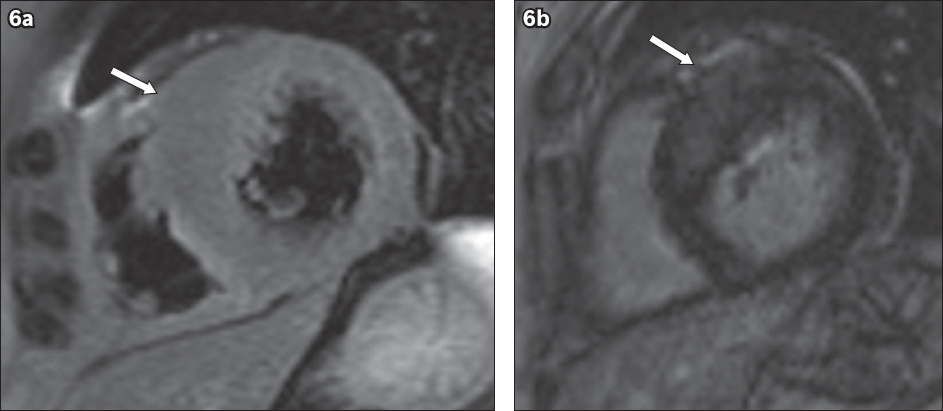
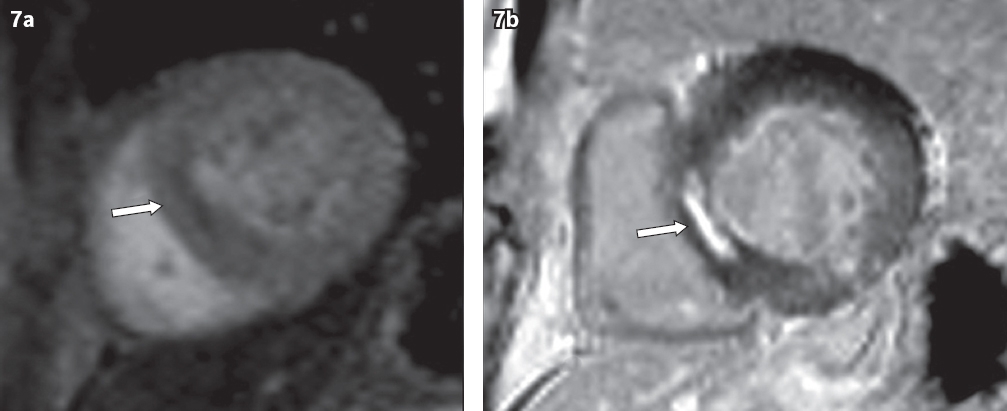
Fig. 7
A 63-year-old man with ischaemic cardiomyopathy. (a) Short-axis perfusion image shows a subendocardial perfusion defect in the interventricular septum (arrow). (b) Short-axis LGE MR image shows LGE in the corresponding subendocardial septum (arrow).
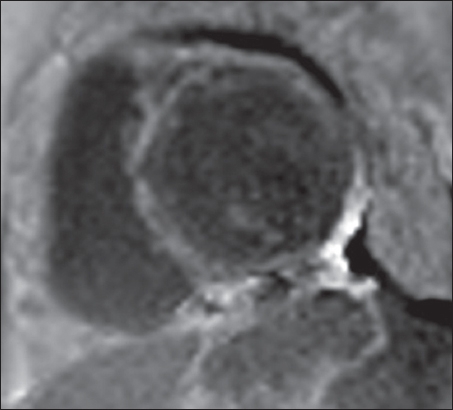
In cardiac amyloidosis, the amyloid protein is deposited in the myocardium and involvement of all four chambers is common, as it is a systemic process. Thus, a specific finding for cardiac amyloidosis on CMR imaging is an increase in the thickness of the interatrial septum and right atrial free wall of more than 6 mm.(7) The most characteristic and distinct imaging pattern of cardiac amyloidosis is global LGE, which is most pronounced over the entire subendocardial circumference (
Fig. 8
A 55-year-old man with amyloidosis. Short-axis LGE MR image shows diffuse subendocardial LGE involving the left ventricle and the interventricular septum.
In summary, CS is not an uncommon entity. When present, it can produce significant symptoms and even sudden cardiac death. Hence, timely diagnosis of CS is essential. However, difficulty arises due to its overlapping clinical manifestations and imaging features with those of other inflammatory and infiltrative cardiac disorders. Imaging diagnosis of CS is challenging. However, in suspected cases, after echocardiography, CMR imaging helps to further characterise the inflammatory and/or fibrotic stages of CS. CMR imaging is also useful in providing comprehensive information regarding the distribution and patterns of cardiac morphological changes and ventricular function. In addition, the LGE phase of CMR imaging is also useful in prognosis and risk stratification.
SMJ-59-412.pdf


