Abstract
A 54-year-old man presented with progressive onset of lower limb paraesthesias, sensory ataxia, gait instability and lower limb weakness. Laboratory findings revealed low serum B12 levels. Magnetic resonance imaging showed long-segment symmetrically increased T2 signal within the dorsal columns of the spinal cord in the lower thoracic spine. The conglomeration of findings was consistent with a diagnosis of subacute combined degeneration of the spinal cord (SCD). Aside from mild residual paraesthesias, the patient’s symptoms largely resolved after treatment with intramuscular injections of vitamin B12. The clinical presentation, pathophysiology, clinical and radiologic differential diagnosis, and management of SCD were described.
CASE PRESENTATION
A 54-year-old Chinese man presented with a three-month history of progressive gait instability, leg weakness, bilateral ascending lower limb paraesthesias and numbness. The patient had no significant past medical history. Neurologic exam revealed 4/5 strength for knee extension and flexion bilaterally. Dorsiflexion and plantar flexion were rated at 4−/5 bilaterally. In addition, proprioception and vibration were markedly diminished in the lower limbs. Babinski reflex was downgoing bilaterally. Laboratory testing revealed macrocytic mean corpuscular volume (103 fL), low serum B12 levels (98 pmol/L), and increased serum homocysteine (25 µmol/L) and methylmalonic acid (310 nmol/L) levels. Bloodwork was negative for anti-intrinsic factor antibodies. What does the magnetic resonance (MR) image (
Fig. 1
MR image of the lower thoracic spine.
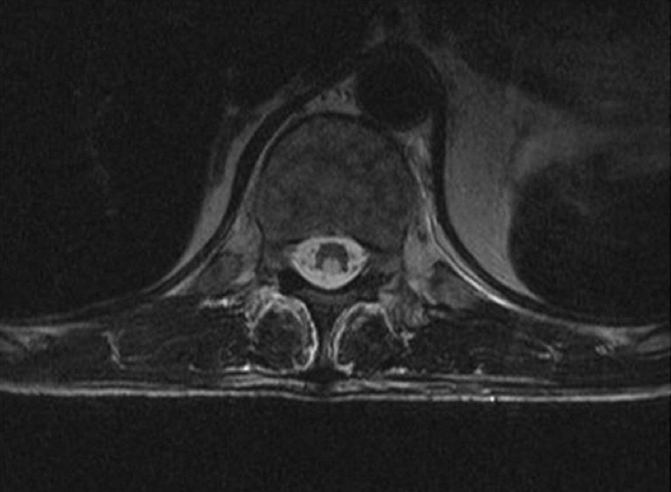
IMAGE INTERPRETATION
Axial MR T2-weighted image shows long-segment symmetrically increased T2 signal within the dorsal columns at the level of the lower thoracic spine. No abnormal enhancement is seen.
DIAGNOSIS
Subacute combined degeneration of the spinal cord (SCD).
CLINICAL COURSE
The patient was admitted to the neurology ward for management and rehabilitation. Treatment was initiated with 1 mg of intramuscular vitamin B12 daily for one week. The patient’s lower limb weakness and sensory ataxia began to resolve, but he was left with mild residual bilateral paraesthesias in his distal toes at the time of discharge. He was given a prescription for weekly vitamin B12 injections to prevent recurrence. At a follow-up appointment with the neurology department several months after discharge, the patient’s symptoms were fully resolved, and he was switched to monthly vitamin B12 injections.
DISCUSSION
SCD is a progressive degeneration of the spinal cord from lack of vitamin B12.(1) It commonly manifests as dysfunction of the dorsal columns (fine touch, vibration and proprioception), and less frequently involves the lateral corticospinal tracts (voluntary motor control of limbs) and lateral spinothalamic tracts (pain and temperature). Clinically, vitamin B12 deficiency may present as paraesthesias, sensory ataxia, gait instability and distal motor weakness, usually in a predominantly lower limb distribution.(2) In more severe cases, spasticity and paraplegia can develop in addition to bladder and bowel incontinence.
Vitamin B12 deficiency can occur due to inadequate consumption of the vitamin or impaired vitamin B12 metabolism and, ultimately, lead to impaired spinal cord myelin synthesis.(1) For example, lack of vitamin B12 can be related to: (a) inadequate intake of vitamin B12 (strict vegetarians); (b) elevated stomach pH (proton pump inhibitors); (c) impaired production of pancreatic enzymes; (d) lack of intrinsic factor (pernicious anaemia); or (e) damage to the distal ileum (inflammatory bowel disease).(1) As vitamin B12 is stored within the liver, the clinical manifestations of deficiency generally occur over a chronic 1–2-year time frame. However, symptoms of SCD can also develop over weeks in the setting of exposure to nitrous oxide gas, due to its ability to inactivate vitamin B12.(3)
While vitamin B12 deficiency is one of the more common causes for dorsal column syndrome, the clinical differential is relatively broad and may include autoimmune, infectious, neoplastic, vascular, metabolic/nutritional and degenerative pathologies.(4) A thorough clinical workup can help differentiate these processes. For example, in addition to symptoms referable to the dorsal columns, multiple sclerosis may also present with vague sensorimotor symptoms and ocular dysfunction including optic neuritis, nystagmus and diplopia.(5) While tabes dorsalis may be associated with tertiary syphilis, it is commonly preceded by local infection of the genitals and multiorgan disease.(6) Spinal cord tumours and spinal dural arteriovenous (AV) malformations have nonspecific clinical presentations; in addition to dorsal column symptoms, patients may have slowly progressive gait disturbances from limb weakness, urinary incontinence, bowel retention and back pain.(7,8) Finally, vitamin E deficiency and hereditary neurodegenerative processes, such as Friedreich’s ataxia, can clinically mimic dorsal column symptoms. However, the former may be associated with chronic steatorrhoea and fat malabsorption,(9) while the latter may also present with cardiomyopathy, diabetes mellitus and a family history of neurodegenerative disease.(4)
With respect to imaging, the MR findings of SCD are almost pathognomonic for this disease, with striking long-segment, symmetric and pyramidal-shaped T2 hyperintensities in the region of the dorsal columns.(10) This signal abnormality is typically nonenhancing, and creates a characteristic inverted V appearance on axial imaging. Very few disease entities will have this classic MR appearance in isolation; acquired immune deficiency syndrome-associated vacuolar myelopathy and copper deficiency are some of the few entities known to be radiologically identical to SCD.(11,12) Demyelination, infection, autoimmune processes, neoplasm and vascular entities can also mimic SCD on MR imaging, yet these processes can potentially be differentiated on close inspection.(11) A recent review of T2 hyperintense spinal cord lesions by Bou-Haidar et al provides a concise outline for their differentiation.(11) For example, demyelination from multiple sclerosis is typically asymmetrical, dorsolaterally positioned within the cord, and less than two vertebral body segments in length (
Fig. 2
(a) A 34-year-old woman presented with vague sensorimotor symptoms and was diagnosed with multiple sclerosis. Axial T2 fat saturated MR image of the cervical spine shows a laterally positioned asymmetric hyperintensity. (b) A 54-year-old woman with a known history of Sjogren’s disease complained of weakness in the lower extremities. Axial T2 MR image of the upper thoracic spine shows a centrally positioned hyperintensity involving more than two-thirds of the cord diameter. (c) A 45-year-old man presented with numbness and tingling in the extremities. Axial T2 fat saturated MR image of the cervical spine shows an expansile, eccentrically-located tumoral cyst and a small crescentic region of haemorrhage along the medial surface of the cyst. Biopsy confirmed that it was an ependymoma.
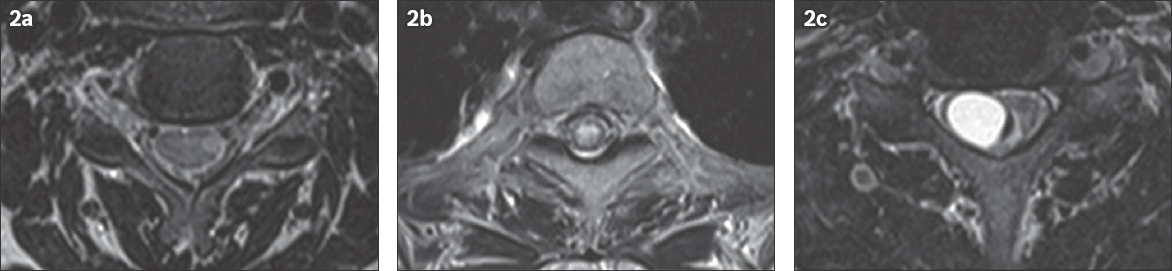
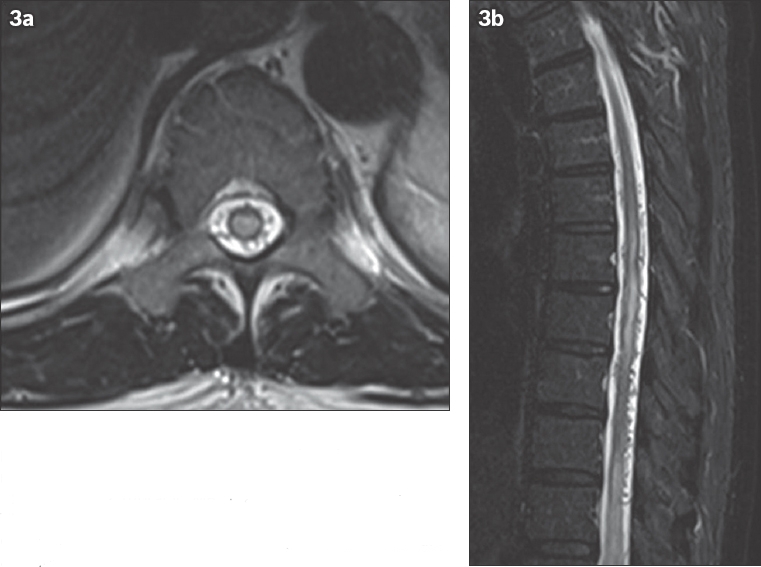
Fig. 3
A 55-year-old man presented with progressive neurogenic claudication and paraesthesia. (a) Axial and (b) sagittal T2 thoracic MR images show central diffuse T2 hyperintensity consistent with cord oedema and dilated collateral veins compatible with a diagnosis of spinal dural arteriovenous fistula.
To clarify the diagnosis, laboratory workup should include a complete blood count with differential, and serum vitamin B12, methylmalonic acid, homocysteine, copper, ceruloplasmin, venereal disease research laboratory, rapid plasma reagin and serum treponemal testing. A complete history-taking and physical examination should be performed to further guide laboratory investigations, such as testing for the presence of anti-intrinsic factor antibodies if pernicious anaemia is suspected, or lumbar puncture with cerebrospinal fluid exam in a patient with a confirmed history of syphilis or clinical suspicion of multiple sclerosis. Ultimately, clinical suspicion of slowly progressive myelopathy necessitates cross-sectional imaging of the spinal cord, and MR imaging is the preferred modality over computed tomography for this indication.(13,14)
The prognosis for SCD patients is largely dependent upon the age and the extent of their clinical disease burden at the time of diagnosis. Patients aged < 50 years with a shorter duration of illness are more likely to have a positive outcome with treatment.(15) Most cases can be fully reversed with intramuscular injections of vitamin B12 over the course of several months. Unfortunately, more advanced cases, such as those progressing to autonomic tract involvement with bladder dysfunction, are less likely to fully recover.(1) With respect to our case, the patient was encouraged to wean himself off his proton-pump inhibitor and increase his dietary intake of animal protein and dairy products.
SMJ-59-464.pdf


