Abstract
INTRODUCTION
This prospective observational study compared the postoperative analgesic effectiveness of intrathecal morphine (ITM) and surgical-site infusion (SSI) of ropivacaine as adjuncts to intravenous (IV) patient-controlled analgesia (PCA) (fentanyl) in living-donor kidney transplant recipients.
METHODS
Patients undergoing living-donor kidney transplantation who received ITM or SSI in addition to IV PCA were included. Rescue analgesia was achieved with IV meperidine as required. The primary outcome, measured using the Numeric Pain Rating Scale (NRS), was pain at rest and when coughing. Patients were assessed for 48 hours after surgery.
RESULTS
A total of 53 patients (32 ITM, 21 SSI) were included in the study. The ITM group showed significantly lower NRS scores, at rest and when coughing, for up to 12 and eight hours. NRS scores were comparable between the groups at other times. The ITM group had significantly less postoperative systemic opioid requirement in the first 24 hours, but there was no significant difference between the systemic opioid consumption of the groups on postoperative Day 2. In the ITM group, 3 (9.4%) patients presented with bradypnoea and 1 (3.1%) with excessive sedation in the first 12 postoperative hours. More patients in the ITM group developed pruritus requiring treatment during the first 24 hours. There were no differences between the groups in other outcomes (e.g. nausea/vomiting, change in pulmonary or kidney functions).
CONCLUSION
Compared with SSI, ITM reduced immediate postoperative pain and IV opioid consumption on postoperative Day 1 after living-donor kidney transplantation, but at the cost of increased pruritus and respiratory depression.
INTRODUCTION
Optimal postoperative analgesia is essential after kidney transplantation, as insufficient pain control can lead to agitation, tachycardia, hypertension and increased risk of pulmonary complications.(1) However, options for post-kidney transplantation analgesia are limited due to comorbidities in kidney transplant recipients and the altered pharmacokinetics of many drugs in these patients.(2)
Although intravenous (IV) opioids are the mainstay of analgesia following kidney transplantation,(3) they do not provide optimal dynamic pain relief after major surgery. Epidural analgesia can offer excellent analgesia,(4) but may be risky in kidney transplant recipients who have undergone dialysis because of platelet dysfunction and residual heparin associated with the dialysis procedure.(5) The combination of intrathecal morphine (ITM) and IV patient-controlled analgesia (PCA) has been demonstrated to be a viable alternative method of analgesia.(6,7) Nevertheless, patients with systemic opioids and ITM have an elevated risk of early or delayed respiratory depression and should be monitored closely.(8)
Blocking of the parietal nociceptive afferent nerves via surgical-site infusion (SSI) of local anaesthetics is currently widely recognised as a useful adjunct to standard analgesic regimens after major surgery.(9,10) Kidney transplant recipients may benefit from SSI with local anaesthetics because kidney transplantation procedures do not usually extend to the intraperitoneal space and the visceral pain component is not involved. The aim of this study was to compare the postoperative analgesic effectiveness and side effects of single-dose ITM and SSI of ropivacaine as adjuncts to IV PCA in living-donor kidney transplant (LDKT) recipients.
METHODS
The institutional review board approved this study in January 2012 and all patients provided written informed consent. The study was conducted from February 2012 to March 2013 at Samsung Medical Centre, Seoul, South Korea. The study protocol was registered with the Korean Clinical Trials Registry (KCT 0000830) after the start of patient recruitment. The authors confirm that all ongoing and related trials for this intervention were registered. Recipients (age range 18–65 years) undergoing LDKT who received either ITM or SSI in addition to IV PCA were included. Exclusion criteria were: allergy to any of the drugs used in the study; contraindication to spinal puncture (e.g. bleeding diathesis, neurologic dysfunction, and recent systemic or local infection); insufficient comprehension to use the PCA device; and history of drug abuse. Additionally, patients receiving opioids for chronic pain were excluded because of potential opioid tolerance or opioid-induced hyperalgesia.(11) This was an open-label study; investigators assessing outcomes were not blinded to the study group assignment, as the authors felt it was unethical to insert an SSI catheter that served no clinical purpose into patients from the ITM group, and patients in both groups were made aware of alternative analgesia regimens.
All patients were instructed about the Numeric Pain Rating Scale (NRS; 0 = no pain, 10 = worst pain imaginable) prior to surgery and on the use of the portable spirometer (Micro Spirometer; Micro Medical Limited, Rochester, Kent, UK). Premedication was not given. Anaesthesia was induced with thiopental (dose 5 mg/kg) and maintained with sevoflurane until retractor application, and desflurane was used thereafter. The depth of anaesthesia in both patient groups was assessed using the Bispectral Index™ monitoring systems (Aspect Medical Systems, Norwood, MA, USA) and maintained within a range of 40–60 to ensure sufficient depth of anaesthesia. Muscle relaxation for intubation was achieved with atracurium (dose 0.5 mg/kg) in both groups. Remifentanil infusion was initiated only when intraoperative systolic blood pressure was above 160 mmHg. One experienced surgeon performed all the operations using the same technique: an inverted J-shaped surgical incision on the lower quadrant of the abdomen just above the groin (hockey stick-shaped incision).
Prior to the induction of anaesthesia, patients in the ITM group received a morphine sulfate injection (dose 400 µg) via the 3th–4th or 4th–5th lumbar interspace using a 27-gauge Whitacre spinal needle after skin infiltration with lidocaine. For the SSI group, the same surgeon inserted a 20-gauge multiholed Soaker catheter (On-Q Painbuster; I-Flow Corp, Lake Forest, CA, USA) 3 cm from the end of the incision after closure of the deep muscle (transversus abdominis and internal oblique muscle) fascia using an introducer needle. The catheters were located in the space along the surgical margin, between the internal oblique and external oblique muscles. After closure of the surgical wound, a bolus dose of 0.75% ropivacaine 10 mL was given and a prefilled elastomeric pump was connected to deliver 0.5% ropivacaine at 4 mL/hour.
After the completion of surgery, all recipients were connected to an IV PCA device (AutoMed 3200; Ace Medical, Seongbuk-gu, Seoul, Korea) that delivers 1,500 µg fentanyl in 100 mL normal saline (dose 15 µg/mL), programmed for a 1-mL bolus, 15-minute lockout time and basal rate of 1 mL/hour. All patients were transferred to the intensive care unit (ICU) and monitored for at least 72 hours, with the instruction to request additional analgesia in case of breakthrough pain. Rescue IV meperidine (dose 50 mg) was administered if the NRS score was more than 5 despite IV PCA (fentanyl) bolus administration, in accordance with the standard protocol at our transplantation centre. The SSI catheter was removed from all LDKT recipients 72 hours after surgery.
The primary outcome evaluated was pain at rest and when coughing, using the NRS. NRS scores at rest and when coughing were assessed at four, eight, 12, 24 and 48 hours after surgery. Secondary outcome measures were: (a) time to first rescue meperidine; (b) number of patients requesting rescue meperidine; (c) consumption of rescue meperidine and IV PCA (fentanyl) at 24 and 48 hours after surgery; and (d) blood urea nitrogen (BUN), creatinine and estimated glomerular filtration rate (GFR) measured immediately after surgery, and on postoperative days (PODs) 1 and 2. All doses of IV opioid consumed were converted to the morphine-equivalent dose to compare systemic opioid consumption between the two patient groups (IV fentanyl 0.1 mg and meperidine 75 mg were considered equivalent to 10 mg of IV morphine).(12)
Respiratory rate and oxygen saturation were measured and recorded every hour in the ICU. Bradypnoea was defined as respiratory rate < 8/minute. The patient’s score on the postoperative sedation scale (1 = completely awake with eyes open; 2 = drowsy; 3 = dozing; 4 = mostly sleeping; and 5 = not responding)(13) and other adverse effects (e.g. nausea/vomiting, pruritus and postdural puncture headache) were evaluated throughout the study period. Rescue medications were chlorpheniramine (dose 4 mg IV) every eight hours for pruritus, and metoclopramide (dose 10 mg IV) every eight hours for nausea. If these proved ineffective, second-line therapy consisted of ramosetron (dose 0.3 mg IV). Each dose administration was initiated at the patient’s request. SSI catheter-related wound complications were assessed for up to seven PODs. Functional vital capacity (FVC) and forced expiratory volume in one second (FEV1) were measured using portable spirometry before surgery and for two PODs.
The normality of continuous data was tested using Shapiro-Wilk test. Normally distributed parameters (e.g. age, body mass index and intraoperative data) were presented as mean ± standard deviation and analysed using Student’s t-test. Non-normally distributed parameters (e.g. NRS score, opioid consumption, sedation score, pulmonary function and perioperative laboratory values) were presented as median (interquartile range [IQR]) and analysed using Mann-Whitney U test. The Bonferroni correction was used for multiple measures. Survival analysis of time until first rescue meperidine was performed using Kaplan-Meier calculations. Categorical data (e.g. presence of nausea/vomiting, low respiratory rate and pruritus) was reported as numbers and percentages, and analysed with chi-square or Fisher’s exact tests, as appropriate. Statistical significance was defined as p < 0.05. All analyses were performed using IBM SPSS Statistics version 21.0 for Windows (IBM Corp, Armonk, NY, USA).
RESULTS
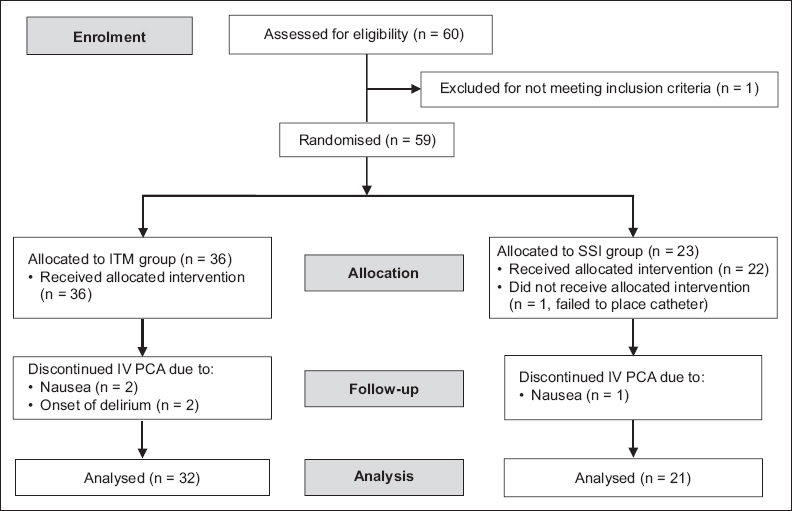
A total of 60 LDKT recipients were screened in our study. After application of the exclusion criteria, one patient was excluded because of chronic opioid use for back pain. Four patients from the ITM group and two from the SSI group were subsequently eliminated for various reasons, and 53 patients (32 ITM, 21 SSI) were included in the final analysis (
Fig. 1
CONSORT flowchart shows the enrolment of living-donor kidney transplant recipients in this study. ITM: intrathecal morphine; SSI: surgical-site infusion; IV PCA: intravenous patient-controlled analgesia
There were no significant differences in the demographic and intraoperative data of the two patient groups (
Table I
Demographic and intraoperative data of LDKT recipients.
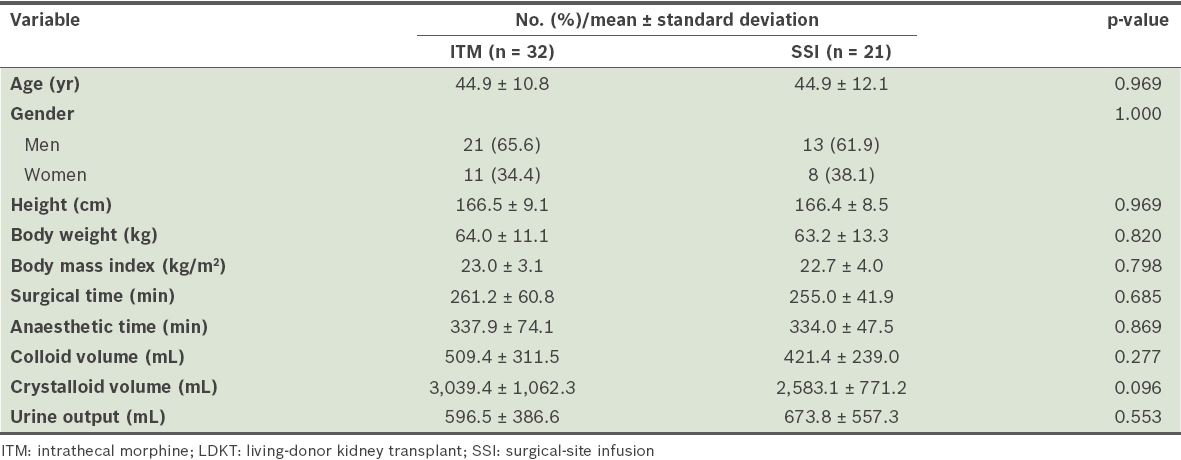
Fig. 2
Chart shows Numeric Pain Rating Scale (NRS) scores at rest and when coughing among living-donor kidney transplant recipients at different times after surgery. Bold lines represent medians, bottom of box indicates the first quartile, top of box indicates the third quartile, and whiskers indicate the minimum and maximum non-distant values. Triangles represent distant or extreme values. *p < 0.05 when compared with the ITM group using Mann-Whitney U test. ITM: intrathecal morphine; SSI: surgical-site infusion
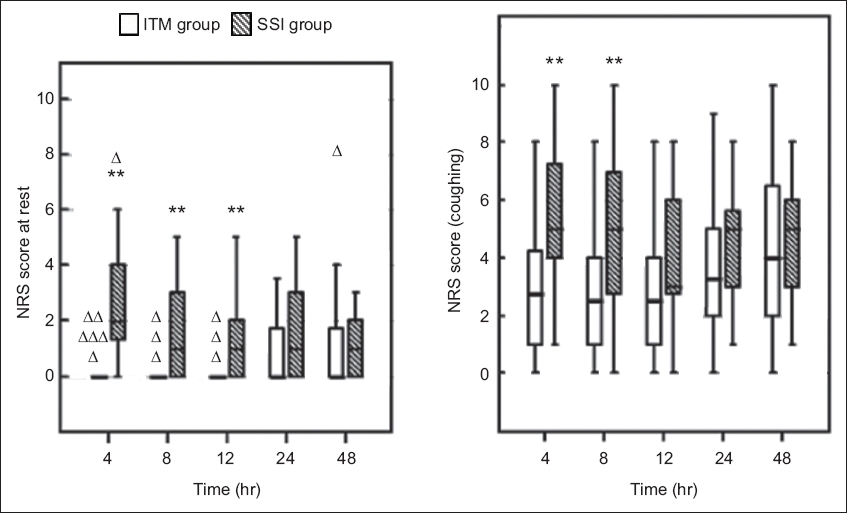
The Kaplan-Meier curves for the two groups showed that ITM was associated with a significant reduction in rescue meperidine use after 12, 24 and 48 hours, as compared with SSI (
Fig. 3
Kaplan-Meier curve shows ratio of living-donor kidney transplant recipients without rescue meperidine at different times after surgery. Significance levels displayed are a comparison of the intrathecal morphine (ITM) and the surgical-site infusion (SSI) groups.
Table II
Supplementary meperidine and IV PCA (fentanyl) requirements of LDKT recipients after surgery.
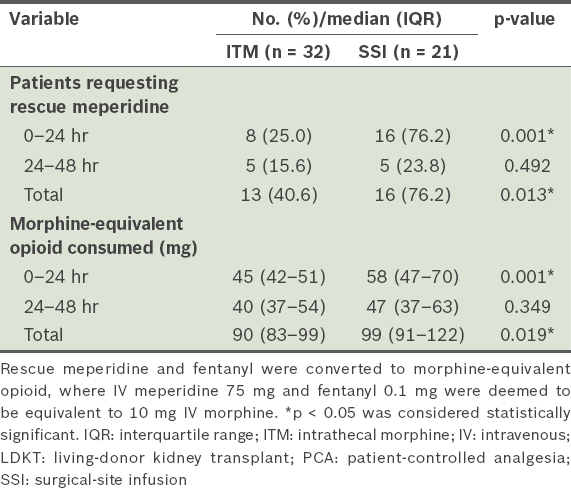
The side effects of analgesia are presented in
Table III
Side effects of analgesia after surgery among LDKT recipients.
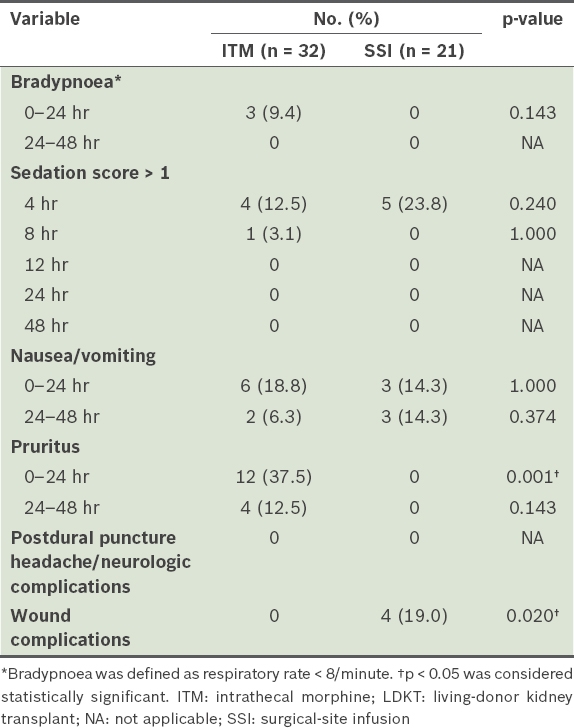
There were no significant intergroup differences in FEV1 and FVC throughout the study (
Fig. 4
Chart shows serial changes in perioperative FVC and FEV1 of living-donor kidney transplant recipients after surgery. Connecting lines represent medians and error bars represent interquartile ranges. FEV1: forced expiratory volume in one second; FVC: functional vital capacity; ITM: intrathecal morphine; preop: preoperative period; SSI: surgical-site infusion
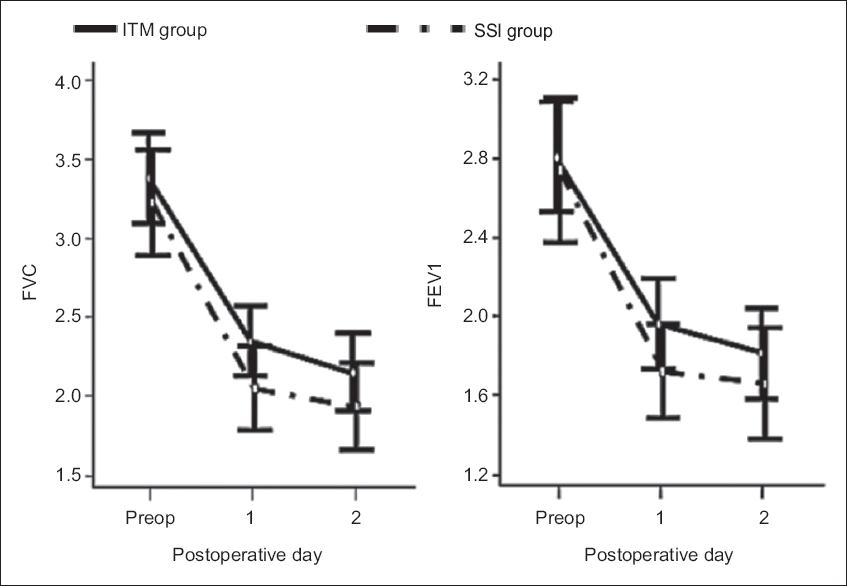
Table IV
Serial changes in perioperative renal function of LDKT recipients.
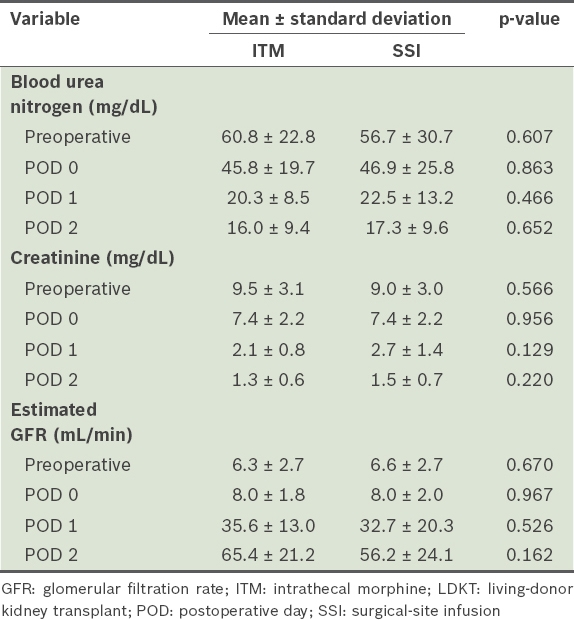
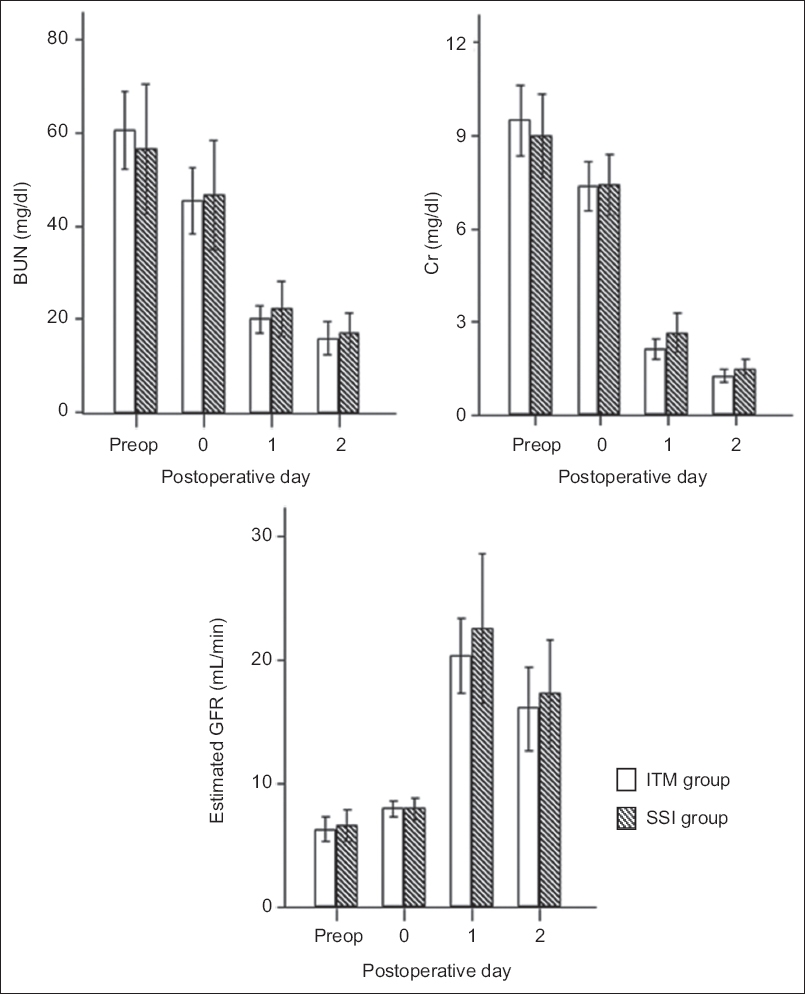
Fig. 5
Charts show serial changes in perioperative blood urea nitrogen (BUN), creatinine (Cr) and estimated glomerular filtration rate (GFR) of living-donor kidney transplant recipients after surgery. Bars represent medians and error bars represent interquartile range. Preop: preoperative period; ITM: intrathecal morphine; SSI: surgical-site infusion
DISCUSSION
Surgery is associated with the stress response, which alters patients’ metabolic and immunological functions.(14) Reduction of the surgical stress response is known to reduce postoperative organ dysfunction.(15) As postoperative pain is widely accepted as a contributing factor to the surgical stress response, optimal postoperative analgesia is essential, especially for immunosuppressed kidney transplant recipients.(16) Hence, the present study compared ITM and SSI of ropivacaine as adjuncts to IV PCA among LDKT recipients. We found that pain relief (assessed using NRS score) and postoperative systemic opioid consumption were significantly better in the ITM group during POD 1, but there was no difference in pain relief between the two patient groups by POD 2. Furthermore, opioid-related side effects, such as pruritus and respiratory depression, were more frequent in the ITM group during POD 1.
ITM is widely employed as an adjunctive analgesic for the treatment of acute and chronic pain, particularly during the perioperative period.(17) The analgesic effect of ITM, combined with IV opioids, lasts for 24–48 hours and is associated with a simultaneous decrease in opioid consumption when compared to IV opioids alone.(6) Lee et al compared the analgesic efficacy of ITM and SSI as adjuncts to IV opioids after hepatectomy and demonstrated that analgesia was more satisfactory during the first 12 hours with ITM.(18) Similarly, the present study showed that ITM reduced immediate postoperative pain and IV opioid consumption during the first 24 hours after surgery when compared with SSI.
However, ITM-related side effects must be thoroughly understood. In our study, the frequency of nausea/vomiting was comparable between the two patient groups. Urinary retention was not assessed because all patients had indwelling urethral catheters throughout the study period. Opioid-related pruritus was more common after neuraxial administration when compared to systemic administration, and pruritus requiring treatment (chlorpheniramine 4 mg IV) was more pronounced for the ITM group. Although most pruritus symptoms in this study were relieved by antihistamines, histamine is not released in intrathecal opioid-induced pruritus and does not appear to be causative.(19,20) Therefore, symptom relief may have been associated with the sedative properties of antihistamines rather than actual relief of the itching sensation. Moreover, antihistamines may enhance the sedative effects of opioids, which could be dangerous when intrathecal opioid is involved.(21) Therefore, opioid antagonists or serotonin Type 3 receptor antagonists, such as ondansetron, rather than antihistamines, should be considered to control ITM-related pruritus.(22)
Delayed respiratory depression is the most critical side effect of ITM and caution should be particularly exercised when adding systemic opioids to ITM because of the potential to increase the risk of early or delayed respiratory depression.(8) Clinical signs of opioid-induced respiratory depression include bradypnoea, low arterial oxygen saturation and decreased level of consciousness. In this study, 3 (9.4%) out of 32 patients in the ITM group presented with bradypnoea and 1 (3.1%) presented with excessive sedation in the first 12 postoperative hours, which was higher than previous reports (range 0%–3%).(6,23) Among them, one patient with excessive sedation required temporary postoperative ventilator support because of hypercarbia. In contrast to our findings, Devys et al reported no bradypnoea following ITM and IV PCA morphine (no background infusion) after abdominal surgery.(6) This discrepancy may be explained by the different IV PCA regimens that were used with ITM in these studies. Since fentanyl has a relatively short half-life, we used IV PCA fentanyl with background infusion and bolus administration. However, this could have caused bradypnoea or excessive sedation when used with ITM. Nonetheless, Devys et al have noted the risk of respiratory depression in patients receiving ITM, as evidenced by hypercarbia two hours after surgery.(6) Consequently, all patients receiving ITM should be monitored for adequate ventilation and consciousness levels.
Given that ITM-induced side effects are dose-related, the minimum effective dose should be prescribed. The ITM dose (400 µg) used in the present study was based on a study by Ko et al.(7) Recent reports have indicated that intrathecal administration of 200–400 µg morphine improved postoperative analgesia without respiratory depression after major abdominal surgery.(6,24) However, the optimal dose of ITM varies according to the specific surgical setting and the patients’ underlying conditions; because kidney transplant recipients have comorbidities and altered pharmacokinetics for many drugs,(2) the ITM dose (400 µg) used in the present study may have been inappropriate. Subsequent to the completion of our study, the pain management protocol at our transplantation centre was changed to a different IV PCA regimen (no background infusion) and lower doses of ITM (dose 200 µg). Further studies on kidney transplant recipients are therefore needed to determine the lowest efficacious dose with adequate analgesic efficacy and minimal side effects.
SSI is widely employed as a useful adjunct during multimodal postoperative analgesia.(25) Its increasing use after major surgery is based on the identification of the important role of parietal nociceptors in pain pathophysiology.(26) In a previous study, the combination of intercostal and ilioinguinal-iliohypogastric nerve blockade decreased both postoperative pain and opioid consumption after kidney transplantation.(27) After the first lumbar nerve (which divides into the ilioinguinal and iliohypogastric nerves) leaves its intervertebral foramen, it runs between the internal oblique and transversus abdominis muscles. At a point approximately two-thirds along this course (near the anterior superior iliac spine), the nerve penetrates the internal oblique muscle to run between the external and internal oblique muscles. We placed catheters in the intramuscular space between the external and internal oblique muscles to decrease pain at these nerve endings.
When compared with a previous study, the use of SSI in the current study did not provide effective postoperative analgesia.(10) Wu et al emphasised the importance of an appropriate concentration and volume of local anaesthetic after prostatectomy.(28) It is possible that our infusion rate (0.5% ropivacaine at 4 mL/hour) was too low to allow sufficient diffusion of the local anaesthetic and thus improve analgesia. However, other studies using similar infusion concentrations and volumes have shown the analgesic benefits of SSI.(10,29) In addition, the effectiveness of the SSI technique may differ according to the placement of the catheters.(25) Previous studies found that introduction of a local anaesthetic agent between the internal oblique and transverse abdominis muscles (i.e. the transversus abdominis plane or TAP) could decrease postoperative pain in various surgery types by blocking the sensory nerves (from the tenth thoracic to first lumbar nerve).(30,31) However, we did not insert a catheter in the TAP because of the need for additional surgical dissection between the transversus abdominis and internal oblique muscles. Furthermore, we were also concerned that the close proximity of the TAP catheter to the transplanted kidney might increase the risk of infection in immunosuppressed recipients. Additional studies should thus address the analgesic efficacy of catheters placed at different sites to determine the optimal catheter location.
Although the safety of using SSI is well established,(29,32) the possibility that SSI of local anaesthetics may increase the risk of wound infection is an important issue, especially for immunosuppressed recipients. In our study, serous oozing at the surgical site occurred in four patients, requiring greater vigilance and regular dressing changes, but did not develop into infection.
There were some limitations to our study. First, the patients were not randomised into defined interventional groups. However, data collection was prospective in nature and all patients received standardised analgesia regimen and postoperative care, reducing the bias that could have arisen from other confounding variables. The demographic factors of the two patient groups were also comparable. Second, both the patients and investigators were not blinded to group allocation (i.e. open-label study), as it was deemed unethical to insert an SSI catheter into patients from the ITM group simply for the purposes of our study. Third, the use of supplementary meperidine may also have been a limitation because its metabolite, normeperidine, is a central nervous system stimulant that may induce seizures and delirium. Normeperidine accumulation may occur in patients with renal failure or graft dysfunction.(33) We were obliged to adhere to the standard pain regimen used at our transplantation centre for this study. However, most recipients received no more than three doses of IV meperidine in 48 hours, and there were no incidences of postoperative graft dysfunction. Finally, we did not evaluate the plasma concentration of ropivacaine. The ropivacaine dose and volume infused in our study was in accordance with research by Forastiere et al (0.5% ropivacaine at 4 mL/hour for 48 hours), and this was well tolerated and not associated with signs or symptoms of toxicity.(10) As only a small fraction of ropivacaine is excreted unchanged into the urine when the liver is functioning normally, its pharmacokinetics are not affected by renal failure.(34) Furthermore, uraemic patients have increased concentrations of plasma alpha-1-acid glycoprotein,(35) which may prevent the accumulation of toxic levels of free or unbound ropivacaine. Therefore, even without measuring plasma ropivacaine concentrations, we were of the opinion that an infusion of 0.5% ropivacaine at 4 mL/hour might be safely instituted in our study.
In conclusion, our study demonstrated that ITM provided better analgesia when compared to SSI as an adjunct to IV PCA for LDKT recipients. However, the short period of increased efficacy (24 hours), and the potential risks of excessive sedation and delayed respiratory depression requiring intense postoperative monitoring should be taken into consideration for patients receiving ITM.


