Abstract
INTRODUCTION
Infective haemodialysis catheter-related right atrial thrombus (CRAT) is a complication of tunnelled catheter use. Management recommendations are based mainly on published case series prior to 2011. We report our institution’s recent experience in managing infective haemodialysis CRAT and correlate treatment with outcomes.
METHODS
We conducted a retrospective analysis of haemodialysis CRAT cases diagnosed on transthoracic echocardiography between 1 January 2011 and 31 December 2017. Clinical outcomes, including mortality at 180 days post diagnosis and thrombus resolution, were traced from electronic medical records.
RESULTS
There were 14 cases identified. The median age was 59 (range 47–88) years and 11 (78.6%) were male. Sepsis was the most common reason for hospitalisation (71.4%). Blood cultures identified Staphylococcus aureus in seven cases, of which two were methicillin-resistant. Three had coagulase-negative Staphylococcus. All cases received antibiotics with infectious disease physician input. Seven were treated with catheter removal alone, of which three died within 180 days. Both cases treated with catheter removal plus anticoagulation survived at 180 days. Of the two cases who had anticoagulation without catheter removal, one died within 180 days and the other did not have thrombus resolution. Three underwent surgical thrombus removal, of which two died postoperatively and the last required repeated operations and prolonged hospitalisation. Mortality at 180 days post diagnosis was 42.9%.
CONCLUSION
Catheter removal and anticoagulation are modestly effective. Surgery is associated with poor outcomes. Despite contemporary management, infective haemodialysis CRAT still results in high mortality. Prospective studies are needed to identify the optimal management.
INTRODUCTION
Tunnelled catheters inserted via the internal jugular or subclavian veins are often used to provide vascular access for haemodialysis in end-stage renal failure (ESRF). The National Kidney Foundation recommends positioning of the catheter tip in the right atrium (RA) to achieve better flow rates.(1) However, this may increase the risk of catheter-related right atrial thrombus (CRAT) formation. This is likely due to repeated catheter tip movement against the RA endocardium, resulting in endothelial damage and activation of the coagulation cascade.(2) As bacteraemia can occur frequently during haemodialysis,(3) the formed thrombus serves as a nidus for infection and becomes an infected vegetation. The optimal management for infected haemodialysis CRAT is unknown and can be either one or a combination of parenteral antibiotics, catheter removal, systemic anticoagulation or surgical thrombectomy. The literature available is limited to sporadic case reports and case series published in 2003–2004.(4,5) With improvements in healthcare delivery, diagnostics, medications and surgical techniques, the outcomes for infected haemodialysis CRAT may have changed. We aimed to describe our centre’s experience with infected haemodialysis CRAT and correlate treatment modalities with outcomes. A brief review of contemporary case reports published in the literature is also presented.
METHODS
We retrospectively reviewed all resting transthoracic echocardiography performed in our institution from 1 January 2011 to 31 December 2017 for cases of right atrial thrombus. Only ESRF cases with either a haemodialysis catheter in situ or documented removal of the catheter during the index admission were included. In addition, all included cases must have at least one positive blood culture for a microorganism consistent with infective endocarditis (IE). Information pertaining to patient demographics, presentation, clinical course, investigations, management and outcomes was collected from the hospital’s electronic medical records. Shapiro-Wilks test was used to check for normality of data, which was then presented as mean ± standard deviation, median (range) and proportion, as appropriate. The primary outcome assessed was mortality at 180 days from echocardiographic diagnosis. The secondary outcome was thrombus size seen on follow-up transthoracic echocardiography within 180 days. Ethics approval was obtained from the hospital’s institutional review board.
RESULTS
We identified 14 cases of infective haemodialysis CRAT, and their characteristics are summarised in
Table I
Demographics, clinical history, presentation and echocardiographic features.
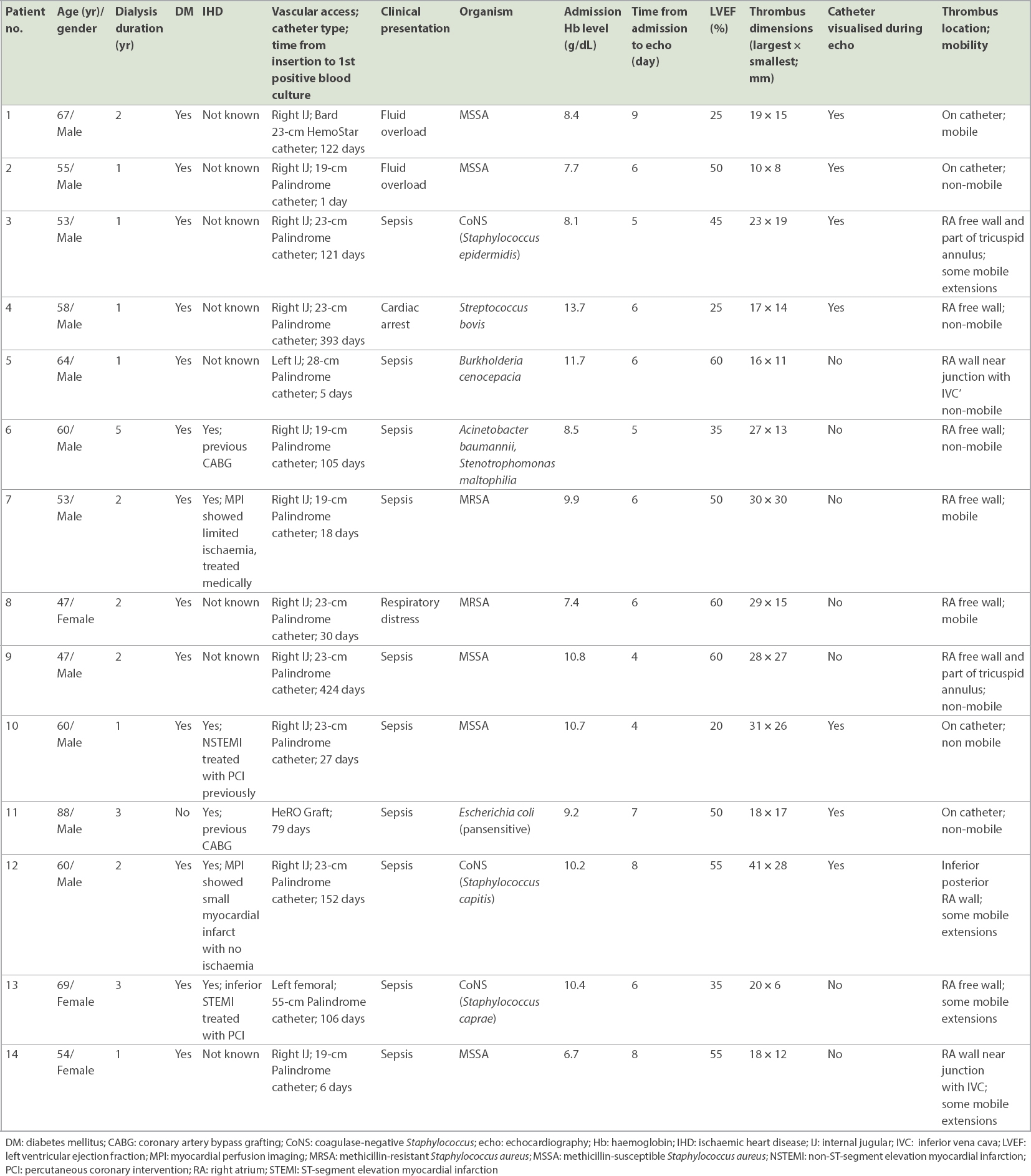
Echocardiographic features of the patients are summarised in
All patients received appropriate parenteral antibiotics guided by blood cultures and input from infectious disease physician. The haemodialysis catheter was also removed during the same admission in 12 cases. Three patients had their catheters removed on the day of the blood culture, with the remaining nine catheters removed at a median of 5 (range 1–7) days after blood cultures were taken. One patient (Patient 10) did not have the catheter removed before death (16th day of admission) due to concerns regarding embolic risk in view of the thrombus extent and attachment to the catheter. The patient with the HeRO Graft had surgical graft explantation 325 days after the index admission, as he was initially deemed to be at high surgical risk.
A summary of the various treatment strategies with their corresponding outcomes is presented in
Table II
Patient outcomes stratified by management strategy.
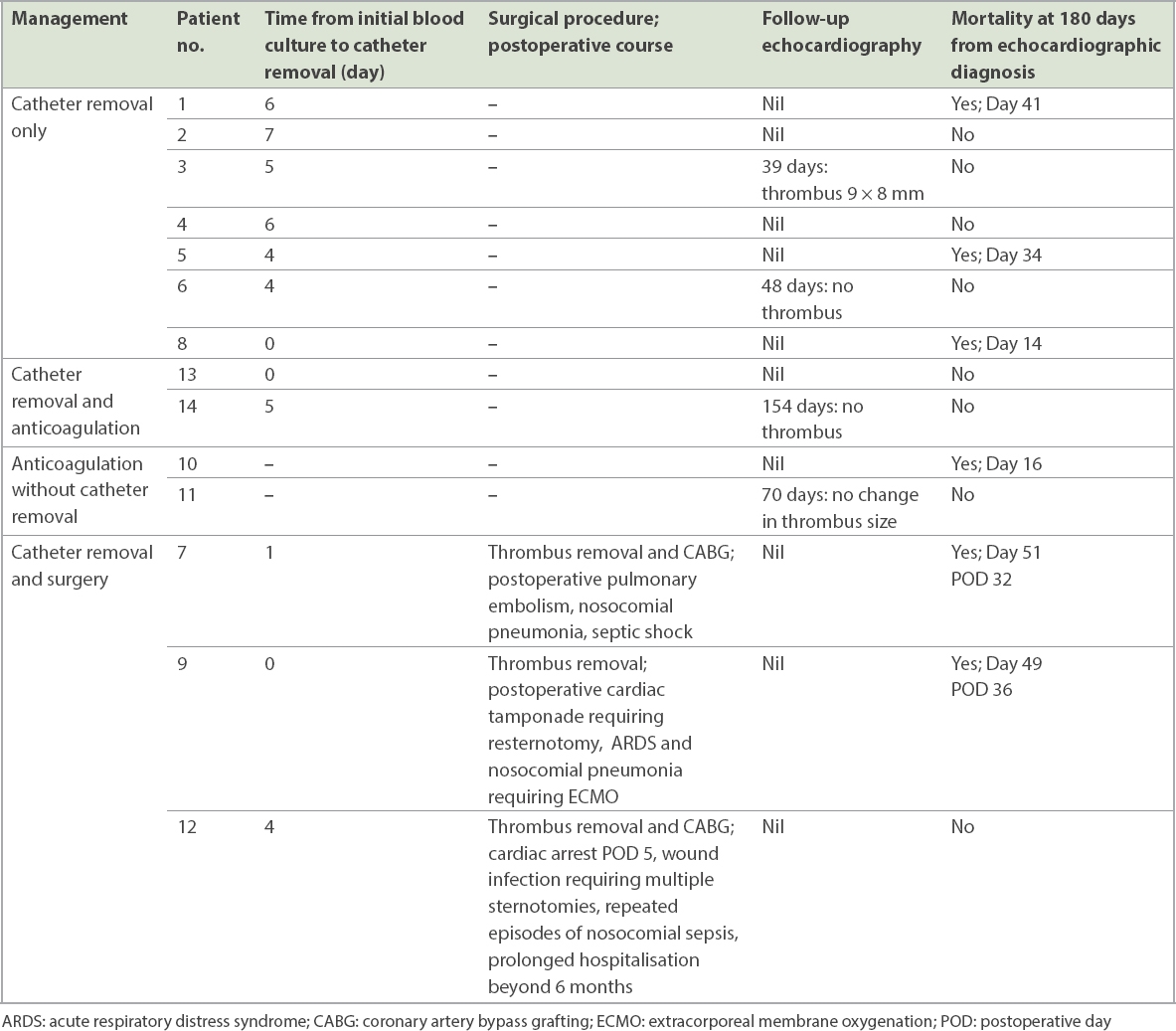
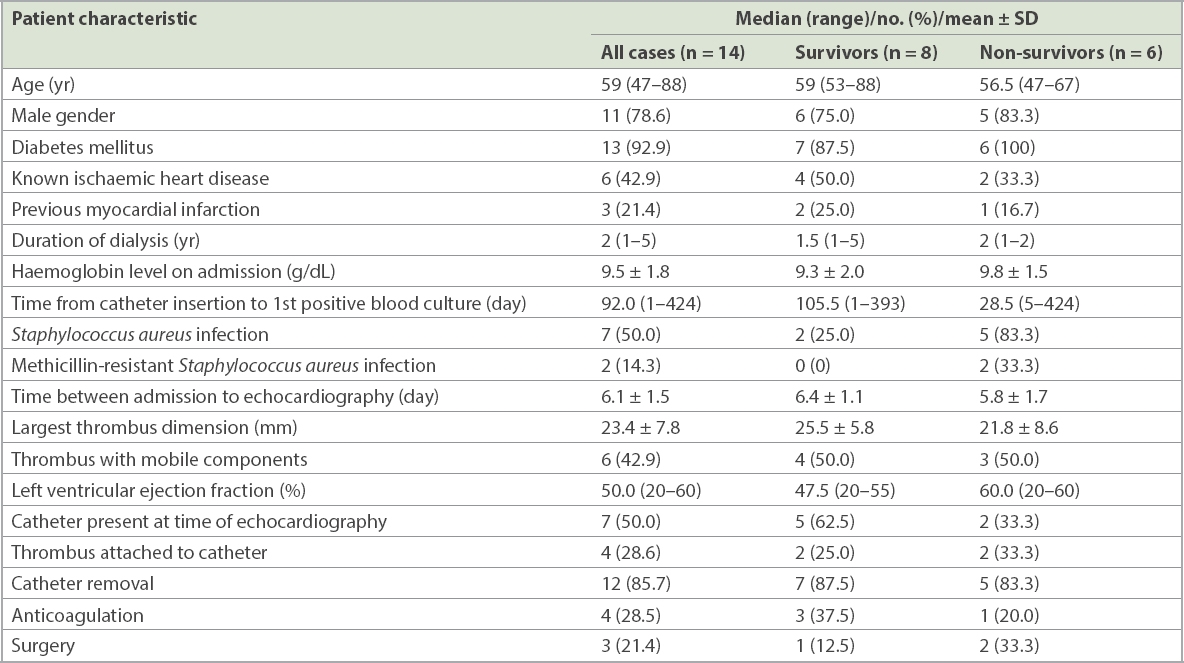
The overall mortality at 180 days post echocardiographic diagnosis was 42.9%. A comparison of the characteristics between survivors and non-survivors is presented in
Table III
Patient characteristics and mortality at 180 days.
DISCUSSION
Patients with ESRF who are dependent on tunnelled catheters for haemodialysis are at risk for infective CRAT for multiple reasons. The positioning of the catheter tip at the level of the RA, as recommended by guidelines,(1) may result in RA wall damage and mural thrombus formation due to constant catheter motion against the endocardium.(7) ESRF patients are also inherently at risk of infection due to uraemia-induced immunodepression.(8) Furthermore, haemodialysis is associated with high rates of bacteraemia,(9) particularly if a catheter, rather than an arteriovenous fistula or graft, is used for access.(10)
There is limited literature on the optimal management of infective CRAT. Current treatment options consist of either one or a combination of antibiotics, catheter removal, anticoagulation and surgical thrombectomy. Existing therapeutic recommendations for CRAT are based solely on retrospective case series in the last decade, and have either included both infective and sterile CRAT(4,11) or evaluated non-ESRF patients with central catheters.(12) Data on infective CRAT published from 2011 and beyond is limited only to isolated case reports.(2,13-16)
Catheter removal is strongly recommended as part of the initial treatment strategy and has been found to influence mortality.(11) In our series, catheter removal alone appears to be modestly effective. Given the potential risk of thrombus dislodgement and subsequent pulmonary embolism, there are published recommendations to keep the catheter in situ initially and only attempt removal when therapeutic anticoagulation has been achieved, particularly for thrombi that are large, mobile or adherent to the catheter tip.(11,12) In our series, this strategy was employed in Patient 10. However, haemodynamically significant pulmonary embolism despite therapeutic anticoagulation has also been reported post catheter removal.(2) Interestingly, none of the patients in our series developed clinical pulmonary embolism, despite the majority having catheter removal during the index admission. There are three possible explanations for this observation. Firstly, our patients had relatively small thrombi that may not have been of sufficient size to cause clinically significant pulmonary embolism, although even catheters with large (5.5 cm) adherent thrombus have reportedly been safely removed without anticoagulation.(17) Secondly, the location of the thrombi may influence the risk of pulmonary embolism. Among the cases with catheter removal, only two had thrombi adherent to the catheter at the time of echocardiography. The thrombi in the remaining cases were mostly attached to the RA free wall, which may result in greater stability as opposed to being on the catheter or at a position closer to the tricuspid valve. Finally, the mobility of the thrombi may also play a role. Half of the thrombi seen were assessed to be non-mobile, with most of the remaining mobile thrombi having only small mobile extensions, which may not result in symptoms even if embolism occurs. These multiple factors contribute to the difficulty in predicting embolic risk and therefore, decisions regarding catheter preservation or removal should be individualised.
Anticoagulation with vitamin K antagonists with a target international normalised ratio (INR) of 2.0–3.0 for six months or until thrombus resolution has been recommended for CRAT.(11) However, only a minority of patients in our series received anticoagulation. Although we were unable to trace the reasons for not initiating anticoagulation in most patients, the most commonly documented reason for withholding anticoagulation was anaemia. Indeed, ESRF results in haemostatic alterations that predispose the patient to bleeding.(18) Observational data extrapolated from the use of warfarin in ESRF patients with atrial fibrillation is conflicting, with some studies demonstrating increased harm from anticoagulation.(18) In light of these concerns, the Kidney Disease: Improving Global Outcomes (KDIGO) consensus statement discourages routine oral anticoagulation in dialysis patients with atrial fibrillation.(19) These concerns regarding bleeding risk, together with the high incidence of anaemia, could explain why only a minority in our series received anticoagulation. Despite this, three out of four anticoagulated patients – including two with catheter removal – had favourable outcomes. This observation is consistent with a previously published case series in which seven out of ten cases of CRAT either resolved or reduced in size with anticoagulation at an INR target of 2.5–3.0, with no reports of bleeding complications.(5) However, anticoagulation, when used alone in our patients, appears to be less beneficial in terms of reducing mortality or thrombus burden. A possible reason is that the catheter continues to serve as a nidus for both infection and thrombus formation, thus negating the effects of antibiotic and anticoagulation therapy.
The role of surgical thrombectomy is not well defined in the literature. A large series consisting of both infective and non-infective haemodialysis CRAT did not show a mortality difference between surgery and anticoagulation.(12) Other authors have reported good surgical outcomes,(16) including the use of minimally invasive surgery.(17) There are several published recommendations supporting the use of surgery for large and/or mobile thrombi.(4,15,16) We have, however, demonstrated very poor surgical outcomes in all three operated cases in our series. This is unlikely to be due to poor patient selection, as all three had under 10% surgical risk estimated using EUROSCORE II and documented clearance of bacteraemia preoperatively. Notably, the EUROSCORE II already includes renal impairment, urgency of procedure, presence of endocarditis and number of procedures (thrombectomy with or without bypass) as variables in the estimation of risk.(7) This may suggest that validated risk calculators for general cardiac surgery may not necessarily apply to patients with infective haemodialysis CRAT, even when patient-specific risk factors are taken into consideration.
Haemodialysis CRAT is associated with significant mortality. A large series of both infective and non-infective CRAT cases encountered up to the year 2010 demonstrated a mortality of 18%.(12) Negulescu et al demonstrated that mortality was 33% in infective cases compared to 14% in non-infective CRAT.(4) Indeed, the mortality rate in our series approximates that of a more recent study of IE in haemodialysis patients, which showed an inpatient mortality of 41%, with an additional 14% mortality within the first year.(20) The high mortality rate among haemodialysis CRAT cases is not surprising given that the known adverse prognostic factors for IE in haemodialysis patients include diabetes mellitus and right-sided IE,(21) which are present in almost all of our patients. Our small patient numbers preclude us from evaluating the variables in
Our study has several limitations. Firstly, the retrospective study design limits our ability to draw comparative conclusions about the various treatment modalities and is highly dependent on the accuracy of the reviewed electronic medical records. The relationship between treatment and thrombus resolution cannot be confidently made, as not all patients had repeat imaging at a pre-determined time interval. Secondly, we were only able to describe a small number of patients in this case series given the rarity of this condition, although to our knowledge, this is the largest case series of infective haemodialysis CRAT reported in this decade. Finally, our cases were collected from a single tertiary medical institution and may not necessarily be universally representative of all patients with infective haemodialysis CRAT.
In summary, our study is the largest case series evaluating the management and outcomes of infective haemodialysis-related CRAT in the current medical literature. Contrary to published recommendations, only a small number of patients received anticoagulation, possibly due to concerns of anaemia and bleeding risk. Most of our anticoagulated patients had favourable outcomes, particularly for those who had catheter removal as well. Surgical outcomes remain poor despite current techniques and should be reserved for selected cases. The overall mortality for infective haemodialysis CRAT remains high despite contemporary management. Although our small series precludes us from identifying patient or treatment variables that are significantly associated with mortality, we have observed a very high proportion of Staphylococcus aureus infection among the non-survivors. Data from further prospective studies is needed to verify this finding and guide us on the optimal management strategy for this group of patients.


