Mdm Chee, a 55-year-old homemaker, visited your clinic for a routine follow-up for her chronic conditions. She shared that she is careful about what she cooks to keep her Type 2 diabetes mellitus, hypertension and hyperlipidaemia controlled. You were happy to note her compliance to medications (metformin 850 mg three times daily, glipizide 10 mg twice daily and amlodipine 10 mg every morning). Her blood pressure was 140/90 mmHg, and her home blood pressure diary revealed similar readings over the past few months. Laboratory results showed a glycated haemogloblin level of 8.0%, serum creatinine of 112 μmol/L (estimated glomerular filtration rate 48 mL/min/1.73 m2) and urine albumin/creatinine ratio of 40 mg/mmol; four months ago, they were 7.8%, 106 μmol/L (51 mL/min/1.73 m2) and 30 mg/mmol respectively. She would soon undergo panretinal photocoagulation on her right eye, but she was scared because the ophthalmologist had said that diabetes mellitus may lead to kidney failure. She asked if there was anything you could do to help her avoid needing dialysis.
WHAT IS DIABETIC KIDNEY DISEASE?
Diabetic nephropathy is traditionally defined as the presence of albuminuria and retinopathy in diabetes mellitus. More broadly, diabetic kidney disease (DKD) refers to the clinical entity of albuminuria, decreased glomerular filtration rate (GFR) or both in diabetes mellitus.
HOW RELEVANT IS THIS TO MY PRACTICE?
Approximately 25%–40% of all patients with diabetes mellitus develop DKD.(1) DKD is the leading cause of end-stage kidney disease (ESKD) in Singapore, with the number of patients initiated on dialysis due to DKD rising 74% from 2009 to 2018.(2) Singapore has the world’s third highest incidence of treated ESKD due to DKD, at 221 per million population.(3) Many more patients with DKD die before they are initiated on dialysis, as DKD is strongly associated with additional cardiovascular mortality.
WHY DO DIABETIC PATIENTS GET KIDNEY DISEASE?
Diabetes mellitus causes kidney damage through complex, overlapping mechanisms.(4) Two key pathways are chronic hyperglycaemia and renin-angiotensin system (RAS) activation.
Chronic hyperglycaemia leads to the accumulation of advanced glycation end-products, reactive oxygen species and inflammatory cytokines. This causes glomerular inflammation, structural changes, such as thickening of the glomerular basement membrane, and mesangial matrix expansion.
Hyperglycaemia increases the amount of glucose filtered at the glomerulus. Correspondingly, sodium-glucose cotransporters (SGLT-1 and -2) are upregulated to reabsorb increased amounts of filtered glucose. This decreases sodium chloride delivery to the distal tubule, which is sensed by the macula densa and results in RAS activation. The combination of afferent arteriole vasodilation and efferent arteriole vasoconstriction increases intraglomerular pressures, causing physical stress to the glomeruli and triggering a profibrotic response.
These processes eventually lead to glomerular sclerosis, albuminuria and kidney impairment.
WHAT CAN I DO IN MY PRACTICE?
As untreated DKD progresses inexorably towards ESKD, the goal of treatment is to prevent or delay the development of ESKD. The role of the family physician is, firstly, to make a timely diagnosis of DKD through screening of individuals with diabetes mellitus and secondly, to retard the progression of DKD through glycaemic control, blood pressure control, RAS blockade, SGLT-2 inhibition and avoidance of further kidney insults. Thirdly, the family physician has an important role in health promotion and in managing comorbidities, especially cardiovascular risk factors.
Screening for diabetic kidney disease
DKD is primarily diagnosed on screening. All diabetic patients should receive annual screening for DKD, beginning five years after the diagnosis of Type 1 diabetes mellitus and at the time of diagnosis of Type 2 diabetes mellitus (as the time of disease onset is unknown). DKD is identified in a diabetic patient by the presence of persistent albuminuria and/or reduced GFR, as well as the exclusion of other causes of kidney disease.
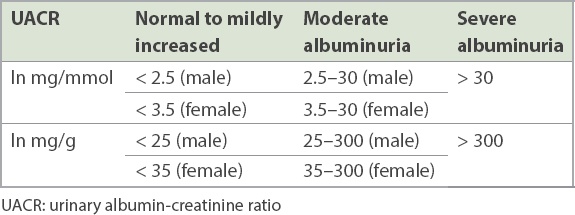
Persistent albuminuria
The preferred proteinuria screen is a spot urinary albumin-creatinine ratio (UACR), which is more convenient than 24-hour urine protein measurement. Urine dipsticks are inadequately sensitive, inadequately specific, and non-quantitative. DKD is diagnosed if the UACR is persistently ≥ 2.5 mg/mmol (25 mg/g) in males and ≥ 3.5 mg/mmol (35 mg/g) in females (
Table I
Categories of persistent albuminuria.
Reduced glomerular filtration rate
GFR can be estimated based on serum creatinine levels. Among the three estimation equations – the Cockcroft-Gault, Modification of Diet in Renal Disease (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations – we favour the CKD-EPI, which is standardised for modern creatinine assays and is most accurate for GFR > 60 mL/min/1.73 m2. Clinicians should consider the limitations of estimation equations, particularly at extremes of age and weight. A sustained reduction in GFR to below 60 mL/min/1.73 m2 for more than three months is consistent with DKD.
Exclusion of other causes of kidney disease
The majority of diabetic patients with kidney impairment have DKD, and kidney biopsy is not routine. However, it is important to consider whether kidney impairment could be due to a process other than DKD. The presence of longstanding, poorly controlled diabetes mellitus makes DKD more likely. This is not always easy to ascertain, as patients with Type 2 diabetes mellitus may have been hyperglycaemic for years before they are diagnosed. On the other hand, kidney impairment in a patient known to have excellent diabetic control for many years, or kidney impairment developing within the first five years of Type 1 diabetes mellitus, may require consideration of causes other than DKD. While the presence of diabetic retinopathy suggests longstanding hyperglycaemia and makes DKD more likely, the absence of diabetic retinopathy does not necessarily rule out DKD.(5,6) Red flags that should prompt a referral to a nephrologist are discussed in the section ‘When should I refer to a specialist?’.
Retard diabetic kidney disease progression
Glycaemic control (target ~7.0%)
In Type 1 diabetes mellitus, there is strong evidence that intensive glycaemic control improves kidney outcomes. The Diabetes Control and Complications Trial demonstrated that intensive glycaemic control early in the course of Type 1 diabetes mellitus (achieving median glycated haemoglobin [HbA1c] level of 7.3% vs. 9.1% in the control group) decreased the long-term incidence of reduced GFR and ESKD.(7)
Studies on Type 2 diabetes mellitus have had mixed results. There is some evidence that intensive glycaemic control (HbA1c 6.4%–6.9%) may reduce the onset and progression of microalbuminuria,(8) while a reduction in long-term incidence of ESKD was seen in only one major study (ADVANCE-ON).(9) Major guidelines state that the HbA1c target in DKD should, in general, be about 7.0%.
HbA1c targets must be individualised; for example, recurrent episodes of hypoglycaemia and older age would prompt the choice of a less stringent target. It is good practice to clearly document the HbA1c target chosen, so that this can be shared with other healthcare practitioners.
Blood pressure control (target < 130/80 mmHg)
The optimal blood pressure (BP) target in DKD is debated. While there is clear evidence of the kidney and cardiovascular benefit of lowering systolic BP below 140 mmHg, further reductions in BP have historically been controversial, with the Kidney Disease Improving Global Outcomes guideline(10) recommending < 130/80 mmHg in albuminuric chronic kidney disease (CKD), while others (such as the Joint National Committee-8 guideline) recommended < 140/90 mmHg. More recently, BP targets have been challenged by the SPRINT trial, which demonstrated the mortality benefit of a systolic BP target of < 120 mmHg versus a target of < 140 mmHg in non-diabetic patients.(11)
Most CKD patients will require more than one, and often three, antihypertensive agents to achieve the target BP. RAS blockade should be the first antihypertensive drug of choice.
Renin-angiotensin system blockade
Angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) have a central role in reversing glomerular hypertension, mitigating albuminuria and slowing the decline in kidney function in DKD, over and above BP targets achieved. The presence of hypertension or albuminuria in DKD is an indication for use of ACE-Is or ARBs (
Table II
Use of angiotensin inhibition in diabetes mellitus subgroups.
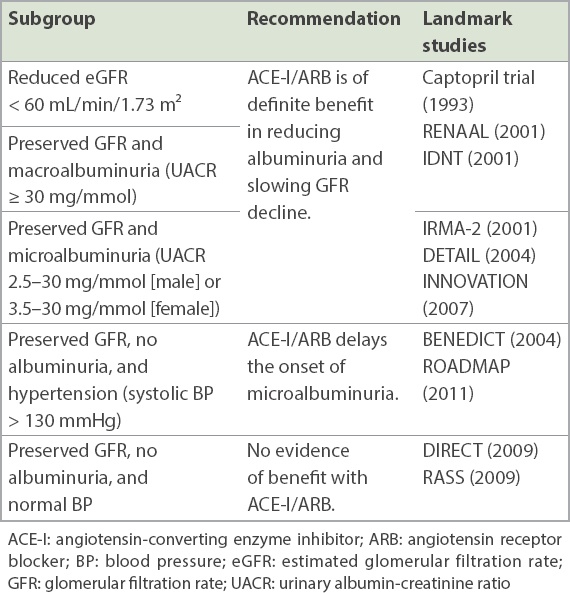
Table III
Recommended doses of common ACE-Is, ARBs and SGLT-2 inhibitors.
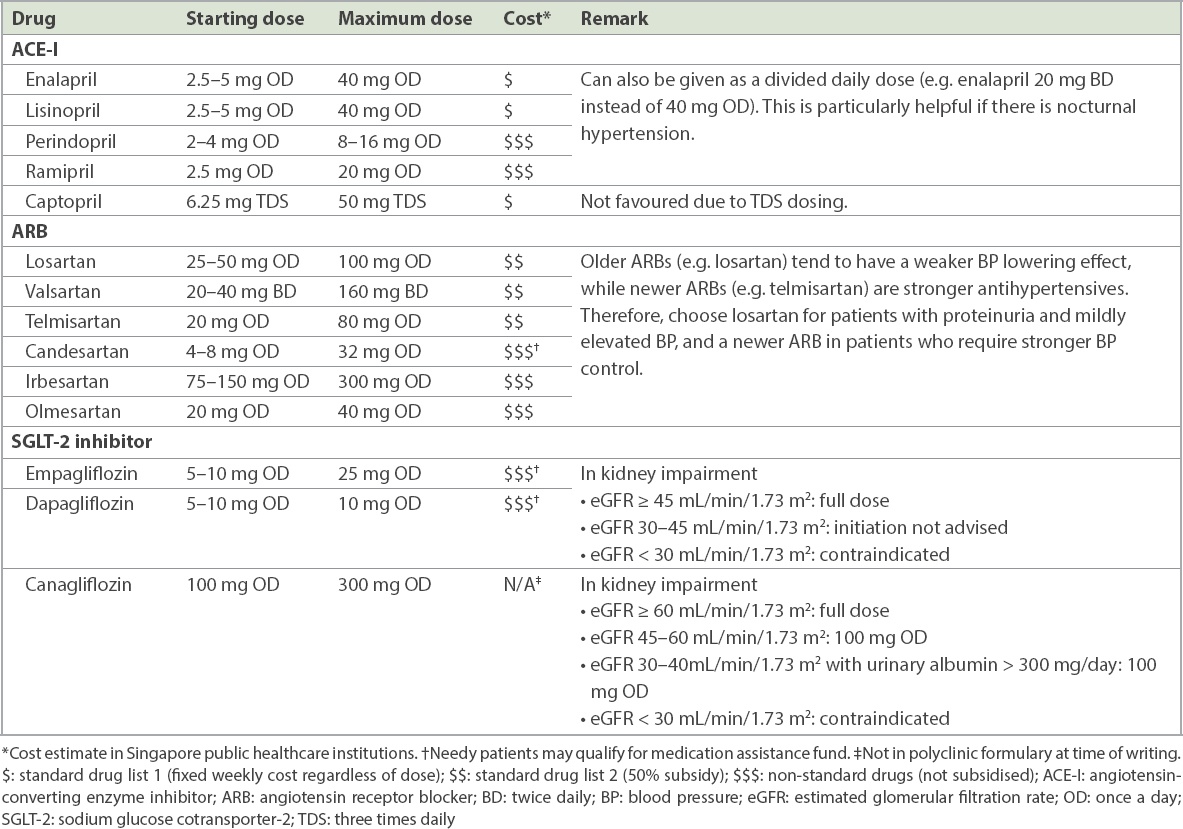
In practice, optimal use of ACE-Is and ARBs in DKD is not always achieved. Important points are as follows:
-
Choose any ACE-I or ARB, start low, and titrate upwards to the maximum tolerated dose. The various ACE-Is and ARBs are considered equally efficacious. If the ACE-I causes cough, switch to an ARB. Optimise the dose by gradual uptitration until (a) the maximum dose is attained, (b) blood pressure is on target and normoalbuminuria is achieved, or (c) further increases are not tolerated (e.g. symptoms of postural giddiness or blood pressure < 120/80 mmHg without any other antihypertensive drugs).
-
Recheck serum potassium and creatinine two weeks after starting ACE-I or ARB or after a dose increase. This is sometimes neglected. ACE-Is and ARBs reduce intraglomerular hypertension via efferent arteriole vasodilation, which slightly reduces GFR and therefore leads to a small rise in creatinine. A rise of 20%–30% is expected. If creatinine rises > 30% or serum potassium rises > 5.5 mmol, stop the ACE-I/ARB and refer to a nephrologist for workup of possible renal artery stenosis.
-
If hyperkalaemia limits ACE-I/ARB dosing, reduce diet potassium before stopping the drug. Hyperkalaemia is often feared and many clinicians discontinue the ACE-I/ARB in the face of hyperkalaemia, denying patients the renoprotective effect of RAS blockade. The first response to hyperkalaemia should be to enforce a low-potassium diet. While temporarily suspending or reducing the dose of ACE-I/ARB may be necessary, every effort should be made to restart it once potassium is controlled. Adding a thiazide or loop diuretic may also be helpful.
-
Reduced GFR is not a contraindication. Absolute contraindications include pregnancy and angio-oedema due to ACE-I/ARB. Some clinicians avoid RAS blockade in patients with reduced GFR. However, patients with reduced GFR benefit most from RAS blockade, which can retard further falls in GFR. The ACE-I/ARB only needs to be stopped in patients who are approaching dialysis dependence.
-
Do not combine ACE-Is and ARBs. Large trials have told us that this is associated with more adverse events of hyperkalaemia and kidney injury without additional clinical benefit.(12,13)
SGLT-2 inhibition
The SGLT-2 inhibitor (dapagliflozin, canagliflozin and empagliflozin) is a promising new drug class that has been shown to retard the progression of diabetic nephropathy. SGLT-2 inhibitors inhibit proximal tubule reabsorption of glucose and sodium, promoting glycosuria and natriuresis, which lowers blood glucose and BP. The increase in sodium delivery to the distal tubules inhibits tubuloglomerular feedback, reducing glomerular hypertension and creating a renoprotective effect. A meta-analysis of the three major SGLT-2 outcome trials (DECLARE-TIMI, EMPA-REG outcome and CREDENCE) showed that SGLT-2 inhibitors reduced major adverse cardiovascular events (myocardial infarction, stroke or cardiovascular death) and slowed the progression of kidney disease.(14)
Guidelines for the use of SGLT-2 inhibitors are evolving. In the CREDENCE trial, canagliflozin was given to DKD patients who had albuminuria (UACR > 300 mg/g) and impaired estimated GFR (eGFR) of 30–90 mL/min/1.73 m2, and were already on ACE-I/ARB treatment.(15) The EMPA-REG trial also included DKD patients with normal GFR ≥ 90 mL/min/1.73 m2 (21% of participants) or no albuminuria (60% of participants).(16) Both trials showed kidney benefit from SGLT-2 inhibition. SGLT-2 inhibitors can be used in DKD patients who have persistent albuminuria in spite of optimised ACE-I/ARB treatment, or who require a second (or third) oral hypoglycaemic agent for suboptimal glycaemic control despite treatment with metformin and lifestyle and dietary modifications. They are not considered first-line therapy at present. Important contraindications and cautions to the use of SGLT-2 inhibitors are:
-
CKD Stage 3B or worse: SGLT-2 inhibitors should not routinely be started when eGFR < 45 mL/min/1.73 m2, and should be stopped if eGFR < 30 mL/min/1.73 m2. (With the CREDENCE trial, it is possible that the eGFR for starting SGLT-2 inhibitor may be lowered to 30 mL/min/1.73 m2.)
-
Previous diabetic ketoacidosis (DKA): euglycaemic DKA has been reported in patients taking SGLT-2 inhibitors, but the absence of hyperglycaemia delays recognition of DKA.
-
Type 1 diabetes mellitus: Key SGLT-2 trials were performed in Type 2 diabetes mellitus patients, and patients with Type 1 diabetes mellitus are at increased risk of DKA.
-
Frequent urinary tract infections: increased rates of genitourinary infections have been reported with SGLT-2 inhibitors.
-
Patients with history of lower limb amputation or at risk of lower limb amputation and patients with peripheral vascular disease or lower limb ulcers: SGLT-2 inhibitors, in particular canagliflozin, are associated with an increased risk of amputation.
With SGLT-2 inhibitors, start low and titrate upwards. In patients with well-controlled diabetes mellitus on insulin, it may be necessary to reduce the insulin dose by 5%–10% to avoid hypoglycaemia. Avoid excessive reductions in insulin dose, as this increases the risk of euglycaemic DKA. As SGLT-2 inhibitors have a diuretic effect, consider decreasing the dose of existing diuretics in patients who are clinically euvolaemic, and reassessing fluid status in 1–2 weeks. Counsel patients on the symptoms of ketoacidosis and risk of urinary tract infections.
Avoidance of further kidney insult
Episodes of acute kidney injury accelerate the progression of CKD and should be avoided.(17) A simple checklist could include:
-
Is my patient’s drug list appropriate for his/her kidney function? Nonsteroidal anti-inflammatory drugs predictably cause acute kidney injury in patients with DKD.
-
Are my patient’s drug doses appropriate for his/her kidney function? As estimated glomerular filtration rate (eGFR) falls, dose reductions or stopping drugs (e.g. metformin) may be necessary.
-
Is my patient taking any potentially nephrotoxic traditional medicine or supplements?
-
Is my patient becoming dehydrated due to diuretics, or SGLT-2 inhibitors?
Health promotion and management of comorbidities
Cardiovascular disease is the main cause of death in DKD. The physician should pay attention to the usual cardiovascular risk-reduction measures, including lipid-lowering and antiplatelet therapy if indicated. Non-pharmacological measures are equally important and must include:
-
Patient education on the course of DKD, common symptoms to expect and measures to retard the progression of DKD
-
Dietary advice, particularly on salt, calorie and glucose intake appropriate to comorbidities
-
Advice on avoidance of nephrotoxic drugs and supplements
-
Vaccinations, including influenza and pneumococcal vaccines
-
Weight management through exercise and dietary measures
-
Smoking cessation
WHEN SHOULD I REFER TO A SPECIALIST?
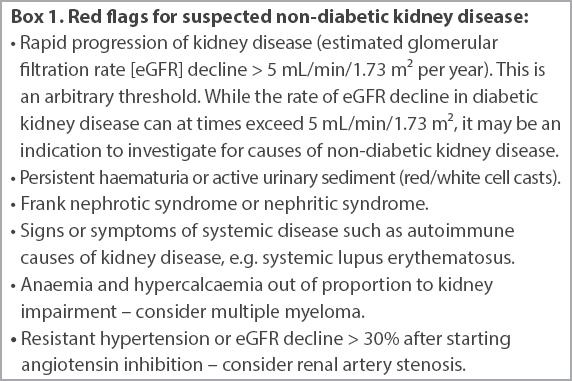
Patients should be referred to a nephrologist for further workup if non-diabetic kidney disease (NDKD) is suspected, or if they are approaching ESKD.
Box 1
Red flags for suspected non-diabetic kidney disease:
Patients approaching end-stage kidney disease
Patients with DKD will face complications or symptoms of kidney failure at eGFR < 30 mL/min/1.73 m2. Most patients will require renal replacement therapy at eGFR < 10 mL/min/1.73 m2. Early referral for elective initiation of renal replacement therapy improves mortality.(18) Preparation for renal replacement therapy typically takes about 12 months, including shared decision-making on the modality of renal replacement therapy, as well as access creation. Therefore, consider a nephrology referral (a) when GFR falls to approximately 30 mL/min/1.73 m2; (b) at higher GFRs if the decline is rapid (> 5 mL/min/1.73 m2 per year); or (c) at any GFR if there are complications that cannot be managed in primary care (e.g. severe anaemia requiring erythropoiesis-stimulating agents, marked hyperphosphataemia or chronic metabolic acidosis).
In the absence of a pressing indication for urgent renal replacement therapy, many patients delay or decline nephrology referral. The family physician should explain that preparing for renal replacement therapy takes time and that planning ahead is preferable to being in a life-threatening situation requiring urgent dialysis. It is often necessary to pace the process with the patient and revisit this conversation at multiple visits. Apart from discussing dialysis planning, it is also important to educate the patient about red flag symptoms that should prompt an immediate emergency department consultation, as well as the importance of fluid restriction and a low-potassium diet.
HOW CAN I MANAGE COMPLICATIONS OF DIABETIC KIDNEY DISEASE?
Complications of DKD that family physicians commonly encounter include fluid overload and mild to moderate anaemia. The family physician may also need to manage symptoms of ESKD in patients who decline dialysis.
Fluid overload
Patients with DKD rarely face overt fluid overload until eGFR falls below 15 mL/min/1.73 m2, in the absence of concomitant heart failure or another cause of fluid overload. The first step in management is to restrict salt (sodium < 2 g/day) and fluid intake. A loop diuretic such as furosemide is usually effective in treating fluid overload. Monitor electrolytes (particularly potassium) with the use of diuretics but avoid routine prescription of supplemental potassium in patients with reduced GFR and baseline serum potassium ≥ 5.0 mmol/L. Thiazide diuretics are ineffective below a GFR of 30 mL/min/1.73 m2 and should be replaced by loop diuretics.
Anaemia
As GFR falls below 30 mL/min/1.73 m2, declining erythropoietin production by the kidneys begins to cause a normocytic, normochromic anaemia. Before attributing anaemia to DKD, exclude other causes by checking iron studies, vitamin B12 and folate, and consider a referral for endoscopy in the presence of iron deficiency anaemia.
In treating anaemia of kidney disease, the first step is to ensure that the patient is iron replete. Initiate iron supplements if there is anaemia with transferrin saturation ≤ 30% and/or ferritin ≤ 500 ng/mL. If anaemia persists after correction of iron deficiency, an erythropoiesis-stimulating agent is indicated, aiming for a haemoglobin target of 10–11.5 g/dL. Erythropoiesis-stimulating agents are generally unavailable in the primary care setting and will require specialist referral.
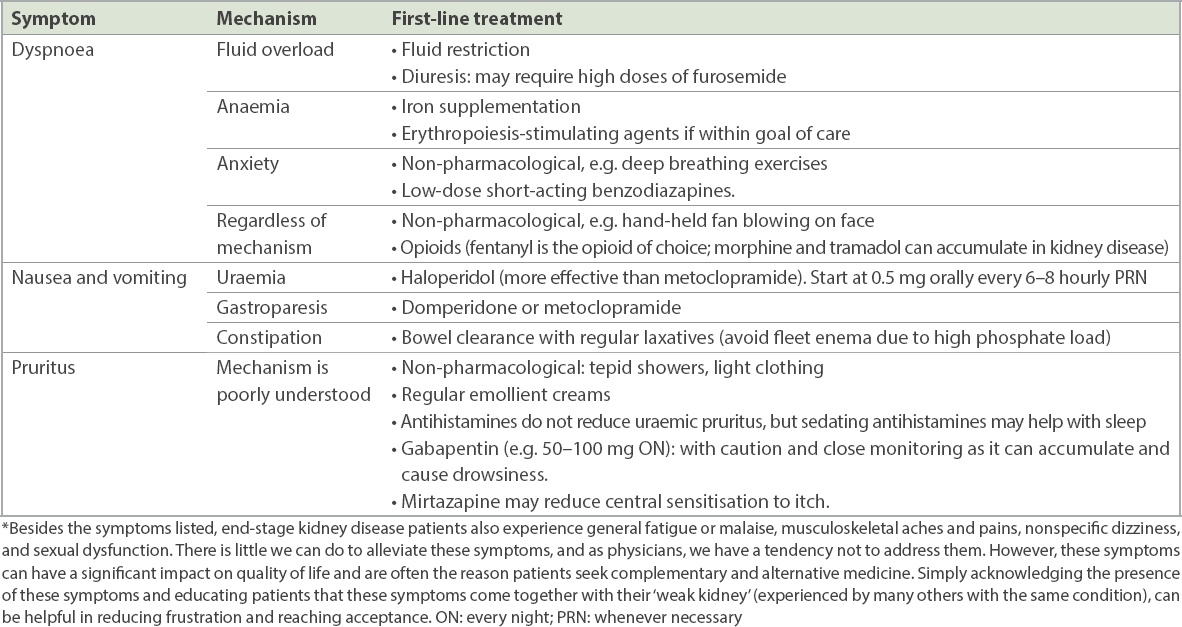
Palliative management of ESKD
Some patients with ESKD decline renal replacement therapy due to advanced age, personal values and preferences, or social constraints. Other patients are unlikely to benefit from renal replacement therapy due to severe life-limiting illnesses or poor premorbid quality of life. Many symptoms of ESKD can be managed in primary care (
Table IV
Palliative management of common symptoms* in end-stage kidney disease.
TAKE HOME MESSAGES
-
All diabetic patients should be screened for DKD, defined by the presence of persistent proteinuria (UACR ≥ 2.5 mg/mmol [25 mg/g] in males or ≥ 3.5 mg/mmol [35 mg/g] in females) and/or reduced GFR.
-
The key objective in the management of DKD is to retard the decline of GFR. This is achieved by optimising glycaemic control (target HbA1c 7.0%), BP control (target BP < 130/80 mmHg in albuminuric DKD) and RAS blockade. SGLT-2 inhibitors are beneficial, particularly in patients with persistent albuminuria, but are avoided if eGFR < 30 mL/min/1.73 m2.
-
Consider referral to a nephrologist when eGFR < 30 mL/min/1.73 m2, in patients with rapid decline of GFR (> 5 mL/min/1.73 m2 per year), or when NDKD is suspected.
-
Preparation for renal replacement therapy can take up to 12 months, and early nephrology referral is associated with better outcomes.
-
Many complications of DKD can be managed in primary care.
You diagnose diabetic kidney disease in Mdm Chee after checking for red flags that were inconsistent with the diagnosis. You started her on linagliptin 5 mg and losartan 25 mg every morning, with a planned review in two weeks to recheck her renal panel and uptitrate losartan. You explained the diagnosis of diabetic kidney disease to Mdm Chee and counselled her on optimal glycaemic and blood pressure control, recommending a low-salt, diabetic diet. Three months later, Mdm Chee’s blood pressure was 128/76 mmHg and her urinary albumin-creatinine ratio had decreased to 10 mg/mmol on losartan 100 mg every morning. Her glycated haemoglobin level was 7.1%, and her creatinine level was stable at 115 μmol/L.
SMJ-61-405.pdf


