Abstract
There has been growing concern surrounding the use of unconfined power morcellation in laparoscopic surgeries for uterine leiomyoma due to its associated risks and long-term clinical sequelae, including parasitic leiomyomas and disseminated peritoneal leiomyomatosis (DPL). We present a case of DPL resulting from previous laparoscopic morcellation and a review of the existing literature. DPL is a potentially devastating consequence of unconfined laparoscopic morcellation in the surgical management of uterine fibroids. A multidisciplinary approach is recommended in the management of DPL, especially in cases of multivisceral involvement. Clinical caution ought to be exercised when using power morcellators; when unavoidable, confined laparoscopic morcellation offers a promising mitigation and should be adopted if practicable.
INTRODUCTION
The advent of minimally invasive surgery (MIS) has brought about improvements in postoperative outcomes in terms of pain control, recovery and scar cosmesis in the surgical management of symptomatic uterine leiomyomas.(1) A major limitation to minimising the size of incisions used in MIS resides in the need for specimen extraction after surgical excision, especially if the specimen is sizeable. This often necessitates an extension of one of the port sites for retrieval. Electric power morcellation was introduced to mitigate this limitation by enabling intracorporeal morcellation of uterine leiomyomas prior to specimen extraction, and was widely adopted as an adjunct to laparoscopic myomectomies and laparoscopic supracervical hysterectomies.(2-4)
Unconfined laparoscopic morcellation is, however, not without its attendant risks and clinical sequelae, including morcellation-related iatrogenic injuries, endometriosis, parasitic leiomyomas, disseminated peritoneal leiomyomatosis (DPL) or even the inadvertent dissemination of undiagnosed leiomyosarcomas.(5-7) These growing concerns led to the United States Food and Drug Administration (FDA) issuing a black box warning highlighting the risk of cancer spread with the use of laparoscopic power morcellators.(8) We present the case of a patient who was treated at our institution for DPL resulting from previous unconfined laparoscopic morcellation and a brief review of the existing literature to caution against the use of unnecessary unconfined laparoscopic morcellation of uterine leiomyomas.
CASE PRESENTATION
A 37-year-old woman with no other significant past medical history had undergone a previous laparoscopic myomectomy with unconfined power morcellation three years ago at an external institution. She presented to our clinic with a one-year history of intermittent lower abdominal pain with recent worsening. Examination revealed a non-tender abdominopelvic mass. Initial investigations, including a urine pregnancy test, were unremarkable. Initial ultrasonography of the pelvis revealed multiple uterine fibroids and a right lower quadrant subcutaneous nodule of similar echogenicity as the uterine fibroids. Computed tomography (CT) of the abdomen and pelvis was subsequently performed for better characterisation of these lesions. The CT coronal image revealed multiple heterogeneously enhancing masses of similar appearance and attenuation in the right subphrenic extrahepatic region, subcutaneous plane of the right lower anterior abdominal wall, lower abdomen and pelvis (
Fig. 1
CT image shows multiple masses in the right subphrenic, lower abdominal and pelvic regions of a patient who presented with disseminated peritoneal leiomyomatosis.
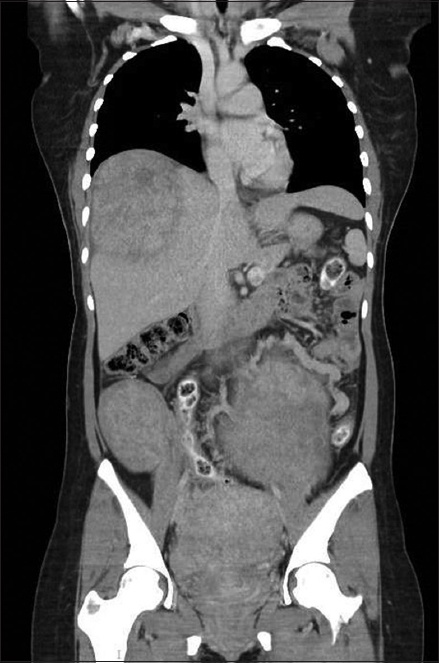
The diagnosis of DPL was established through a high index of clinical suspicion based on the patient’s history of previous laparoscopic power morcellation for uterine fibroids, suggestive CT findings and a subsequent excisional biopsy of the abdominal wall lesion, which revealed a benign leiomyoma.
CLINICAL COURSE
Following extensive multidisciplinary discussions and patient counselling, preoperative angiography and embolisation was planned, followed by definitive surgical excision of the DPL. During preoperative angiography, embolisation of the main feeding vessels to the right subphrenic lesion (branches of the right hepatic, right inferior phrenic and right posterior intercostal arteries) and pelvic lesions (branches of the inferior mesenteric and bilateral internal iliac arteries) was carried out. The patient subsequently underwent laparotomy for excision of her multiple peritoneal leiomyomas. Several concomitant procedures were required, including a total hysterectomy, bilateral salpingectomy, left oophorectomy, high anterior resection, resection of a cuff of diaphragm and dissection of the right subphrenic lesion off the liver.
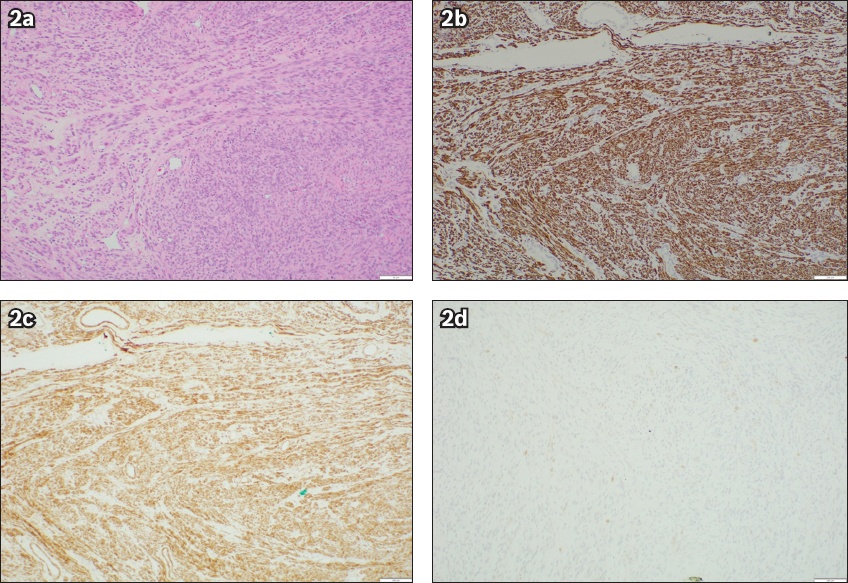
Final histological examination confirmed the diagnosis of benign leiomyomas in all resected specimens (
Fig. 2
(a) Haematoxylin and eosin-stained section of the left pelvic leiomyoma shows fascicles of eosinophilic spindle cells (× 100). Immunohistochemical stains show positivity to (b) desmin (× 100) and (c) caldesmon (× 100), and (d) negativity to CD117 (× 100).
DISCUSSION
Parasitic leiomyomas and the more extensive sequelae of DPL have long been reported in the literature to occur either de novo or following conventional myomectomy or hysterectomy procedures.(9,10) However, the number of cases of parasitic leiomyomas and DPL reported has increased significantly following the introduction of laparoscopic power morcellation.(9,10) We have encountered two cases in our practice thus far, one of which is illustrated in this case presentation.
The incidence of parasitic leiomyomas and the more extensive sequelae of DPL following laparoscopic morcellation is estimated at up to 0.95% and 0.35%, respectively.(9,10) Although DPL is relatively uncommon, it has devastating clinical implications, as it tends to occur in women who are relatively young (mean age 40.8 years at diagnosis) and requires extensive excisions, frequently involving multivisceral resections with significant complications and morbidity.(11)
DPL takes an average of 39–132 months to present after initial laparoscopic morcellation, with a mean number of 2.9 lesions (range 1–16) at presentation.(11,12) Unconfined morcellation has been strongly suggested to be a risk factor for the subsequent development of parasitic leiomyomas and DPL.(11) During unconfined morcellation, small leiomyoma fragments and microscopic deposits, which harbour the potential for regrowth into parasitic leiomyomas, are easily dispersed and lost within the peritoneal cavity.(11) The exact pathophysiology behind DPL is postulated to involve a complex interplay among individual patient genetic predispositions, subperitoneal mesenchymal stem cell metaplasia and hormonal influences, abetted by iatrogenic seeding of the abdominal cavity with leiomyoma fragments from unconfined morcellation. Even with extensive peritoneal lavage, it is becoming increasingly clear that surgical removal of tissue fragments arising from unconfined morcellation is often incomplete and that they do not invariably disintegrate in vivo.(9)
Multidisciplinary approach to management
The management of DPL, not unlike the surgical management of peritoneal carcinomatosis, requires meticulous excision of all visible leiomyoma deposits, with or without en bloc multivisceral resections to achieve adequate clearance. In light of this, a multidisciplinary approach across various surgical disciplines may be necessary and recommended. Even after upfront extensive surgical resection, due to its disseminated nature and inability to ensure complete disease clearance at the microscopic level, DPL has been reported to recur, as seen in our case, and may necessitate repeated surgical interventions.(9) Additionally, patients may suffer long-term sequelae from the morbid surgical resections required in the treatment of DPL, including adhesion-related complications (e.g. adhesion colic, adhesive intestinal obstruction), incisional hernias (especially when en bloc abdominal wall resections are required for parasitic deposits) and possibly short gut syndrome in cases of extensive bowel resections.
There is currently no consensus on the role of ‘adjuvant’ treatment for DPL following surgical excision due to the relatively paucity of reported cases. Leuprorelin, a gonadotropin-releasing hormone (GnRH) agonist, is the only FDA-approved pharmacological agent for the management of symptomatic uterine fibroids. Chronic administration of a GnRH agonist results in a physiological downregulation of GnRH receptors, thereby decreasing hypothalamic secretion of gonadotropins, achieving a reduction of serum oestrogen to post-menopausal levels. The use of leuprorelin in the ‘adjuvant’ treatment of DPL as a pharmacological extension of its established use in the medical management of symptomatic uterine fibroids (as in our patient) has been reported previously.(13)
Contained power morcellation
In a bid to circumvent the problem of peritoneal dissemination while preserving the perioperative benefits of an MIS approach in the management of uterine leiomyomas, several authors have published their experience with contained (‘in-bag’) morcellation techniques.(14) Studies comparing the conventional unconfined version with this newer contained technique of power morcellation mostly report similar intraoperative blood loss, postoperative complication rates and length of stay, with the downside of a slightly longer average operative duration of 17–26 minutes when compared with the contained technique.(14) The slightly longer operative duration may in part be related to a surmountable initial lack of familiarity with the contained technique and may be worthwhile to minimise the risk of the sequelae of DPL down the line.
In conclusion, DPL is a potentially devastating consequence of unconfined laparoscopic morcellation in the surgical management of uterine fibroids. A multidisciplinary approach is recommended in the management of DPL, especially in cases of multivisceral involvement. Clinical caution ought to be exercised when using power morcellators; in cases where it is unavoidable, confined laparoscopic morcellation offers a promising mitigation and should be adopted whenever possible.


