Abstract
INTRODUCTION
Serum creatinine is crucial in glomerular filtration rate (GFR) estimation. Various methods of measuring GFR have been developed, which vary in their ability to estimate the prevalence of chronic kidney disease (CKD) and predict consequences associated with CKD. The use of different laboratory devices also results in uncertainty in estimated GFR (eGFR). The purpose of our study was to discuss the effect of differences in laboratory devices on eGFR when performing serum creatinine measurements.
METHODS
163 participants aged 51.22 ± 18.66 years were enrolled during a community health screening programme conducted on 18 June 2011. Samples were sent to four different hospitals using four different devices to check serum creatinine by the Jaffe and enzymatic creatinine methods.
RESULTS
Using Roche Cobas Integra 400, Beckman LX20, Hitachi 7180 and Toshiba TBA – c8000, the proportion of the population with eGFR < 60 mL/min/1.73 m2 was 11.04%, 6.75%, 20.25% and 20.86%, respectively. Moreover, 3.68% of the participants had eGFR < 60 mL/min/1.73 m2 in the laboratory when Roche Cobas Integra 400 was used with the enzymatic creatinine method and compensated Jaffe method.
CONCLUSION
Although standardisation of serum creatinine measurement has been achieved by using isotope dilution mass spectrometry, differences in measurement devices still cause substantial bias in the overall results. This affects the application of GFR in the estimation of CKD progression and outcomes associated with CKD.
INTRODUCTION
Chronic kidney disease (CKD) is a global health concern. Numerous studies have investigated the association of CKD with end-stage renal disease (ESRD), increased mortality and cardiovascular diseases.(1) The expression of CKD varies with its aetiology, severity and rate of progression.(2) The United States Renal Data System showed that Taiwan has the highest frequency of dialysis usage and incidence rate of CKD.(3) According to the Taiwan Renal Data System, the overall prevalence of ESRD increased gradually from 368 per million people in 2001 to 2,447 per million people by 2009.(4) A high prevalence of CKD and lack of awareness among the population have burdened the Taiwan’s National Health Insurance Administration. Therefore, a serum creatinine (Scr)-based prediction equation to calculate estimated glomerular filtration rate (eGFR) is crucial for the early diagnosis of CKD.
The classification of CKD depends on eGFR values.(5) This predictive value is estimated using an equation containing several variables including Scr level, age, gender and race to estimate the GFR per minute. Scr is calculated using the conventional unit (mg/dL). The current Modification of Diet in Renal Disease (MDRD)-GFR equation is as follows: 186 × (Scr)−1.154 × (age)−0.203 × (0.742 if female) × (race factor).(6) The most common measurement used to estimate GFR is Scr level. Currently, the Jaffe reaction and enzymatic creatinine method constitute the two major assays used for estimating Scr level.(7) The Jaffe reaction is susceptible to interference from non-creatinine chromogens such as blood sugar, protein, uric acid, ketone bodies and cephalosporin,(7,8) resulting in overestimated Scr values. Overestimation of Scr by the Jaffe reaction was observed in approximately 20% of people with normal renal function.(9) In patients with ESRD, interference from non-creatinine chromogens is less evident because of the extremely high values of Scr, thus making overestimation more prominent in patients with normal renal function.(9) Compared to the Jaffe reaction, the enzymatic creatinine method is less affected by interference from non-creatinine chromogens and more accurate in measurement,(9,10) but it is more expensive and less applicable in clinical practice. The nonequivalence among the Scr assays results in high variability of Scr values and can substantially affect eGFR accuracy. This variability in Scr assay results among laboratories is well known, and calibration is necessary for ensuring accurate results.(11)
In 2006, the United States’ National Kidney Disease Education Program (NKDEP), International Federation of Clinical Chemistry and Laboratory Medicine, United States’ National Institutes of Health and European Communities Confederation of Clinical Chemistry suggested an inter-laboratory calibration for Scr measurement.(12,13) Isotope dilution mass spectrometry (IDMS) is an internationally recognised method that uses the following MDRD-GFR equation for estimating GFR:(14) 175 × (Scr)−1.154 × (age)−0.203 × (0.742 if female) × (race factor). The use of IDMS reduces inter-laboratory variability and provides higher accuracy in the estimation of GFR.(13,14) However, the accuracy of GFR estimation is liable to be affected by variability such as differences in measurement devices, methodology and calculation as well as interference by chromogens or nonspecific proteins in serum. Hence, in this study, we compared Scr results obtained using different devices and assays, and evaluated the effects of these differences on eGFR accuracy.
METHODS
A total of 163 participants aged 51.22 ± 18.66 years from Mudan Township, Pingtung County, Taiwan, were enrolled at a community health screening programme conducted on 18 June 2011. Their blood samples were collected between 0800 hours and 1100 hours. The participants were asked to fast for at least eight hours before blood sampling. Each participant’s blood was drawn using a vacutainer serum-separating tube and a blood collection tube, and the samples were stored at 4°C–8°C in a container before they were sent to a laboratory for examination. The samples were centrifuged at 3,000 revolutions per minute (rpm) for ten minutes. The serum was collected, divided and stored in 2-mL plastic containers. All specimens were stored at −20°C before further examination of Scr.
The samples were sent to four different laboratories with four different types of equipment to estimate Scr values using the Jaffe reaction and enzymatic creatinine methods. Samples were centrifuged at 3,000 rpm for five minutes. The serum was collected for analysis according to standard operation and inspection processes. To optimise Scr accuracy, samples were analysed for three days according to NKDEP creatinine standardisation guidelines(15) using the Jaffe reaction and enzymatic creatinine methods. The chromogen used was calibrated through IDMS.
The study protocol was approved by the institutional review board of the Kaohsiung Medical University Hospital (KMUH-IRB-980457), Kaohsiung, Taiwan. Informed consent was obtained in written form and all clinical investigations were conducted according to the principles in the Declaration of Helsinki. The patients gave consent for the publication of clinical details.
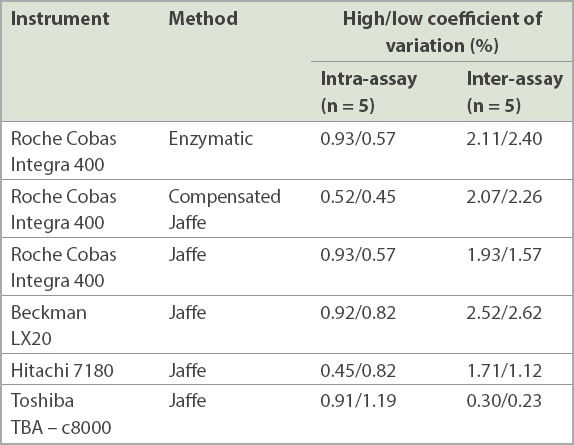
The following devices that are often used in Taiwan were employed for this analysis – (a) Roche Cobas Integra 400 (Roche Diagnostics, Rotkreuz, Switzerland), reagent: Cobas Integra 400 Creatinine Plus Version 2 and Jaffe Gen.2 (Roche Creatinine plus/Roche Diagnostics GmbH/Mannheim, Germany), standardised calibration reference materials: National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 914, proficiency testing: Bio-Rad External Quality Assurance Services [EQAS] assessment, where all EQAS programmes had American Association for Laboratory Accreditation ISO/IEC 17043:2010 Conformity Assessment – General Requirements for Proficiency Testing certification; (b) Beckman LX20 (Beckman Coulter, Brea, CA, USA), reagent: SYNCHRON® Systems (MUS3803000 Beckman Coulter Inc, Carlsbad, CA, USA), standardised calibration reference materials: CX/LX/LXi system analytical software, proficiency testing: College of American Pathologists assessment; (c) Hitachi 7180 (Hitachi, Tokyo, Japan), reagent: Wako (MJP1383000 Fujifilm Wako Pure Chemical Corporation Mie Plant, Mie-gun, Mie, Japan), standardised calibration reference materials: NIST SRM 914a, proficiency testing: Taiwan Accreditation Foundation (TAF) assessment; and (d) Toshiba TBA – c8000 (Toshiba, Tochigi, Japan), reagent: Denka Seiken (MJP0394000 Denka Seiken Co Ltd, Niigata, Japan), standardised calibration reference materials: NIST SRM 914a, proficiency testing: TAF assessment.
Analysis of variance was used for data analysis (Prism 5; GraphPad Software Inc, La Jolla, CA, USA). All values were expressed as mean ± standard deviation. The relationship between quantitative values were expressed using r2, with an r2 value nearer to 1 indicating stronger correlation. A value of p < 0.05 was considered significant. Scr level was expressed in mg/dL. eGFR was calculated using the following IDMS MDRD equation: 175 × (Scr)−1.154 × (age)−0.203 × (0.742 if female) × (race factor).(16)
RESULTS
To ensure the precision of the analyser, pooled serum samples with known values of high creatinine level (Scr = 1.71 mg/dL) and low creatinine level (Scr = 0.55 mg/dL) were analysed in four different devices. During analysis of the variation (n = 5), the device was in a stable condition with the coefficient of variation being less than 5% (
Fig. 1
Graph shows the effect of measurement devices and methods on estimated glomerular filtration rate (eGFR) measurement. R-E: Roche (Cobas Integra 400) enzyme; R-J-C: Roche (Cobas Integra 400) compensated Jaffe; R-J: Roche Jaffe; B-J: Beckman (LX 20) Jaffe; H-J: Hitachi (7180) Jaffe; T-J: Toshiba TBA – c8000 Jaffe
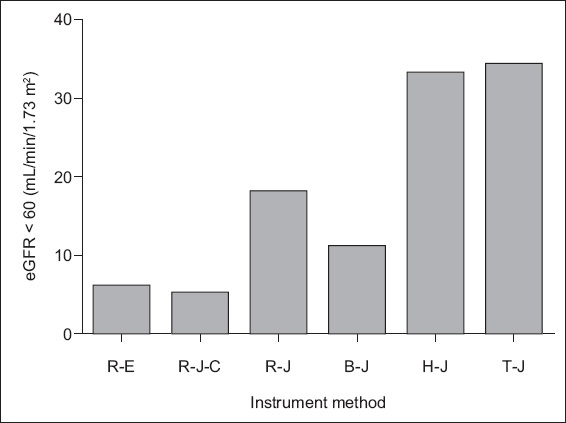
Table I
Value of the coefficient.
We selected the enzymatic creatinine method, which is standardised against IDMS as the gold standard, to investigate the difference in Scr measurement by the Jaffe reaction after standard calibration by IDMS, because of its higher specificity and freedom from interference by bilirubin and other medication. The results of Scr bias obtained using the Roche enzymatic, Roche compensated Jaffe, Roche Jaffe, Beckman Jaffe, Hitachi Jaffe and Toshiba Jaffe methods were −0.008 ± 0.02, −0.165 ± 0.05, −0.026 ± 0.07, −0.18 ± 0.08 and −0.223 ± 0.06, respectively (Figs.
Fig. 2
(a) Scatter graph shows the correlation between the Roche Cobas Integra 400 enzyme creatinine (Roche-E-Cr) and Roche compensated Jaffe creatinine (Roche-J-C-Cr) methods (n = 163, r2 = 0.9913, p < 0.0001). (b) Bland-Altman plot shows agreement between the two methods (bias = −0.08 ± 0.02).
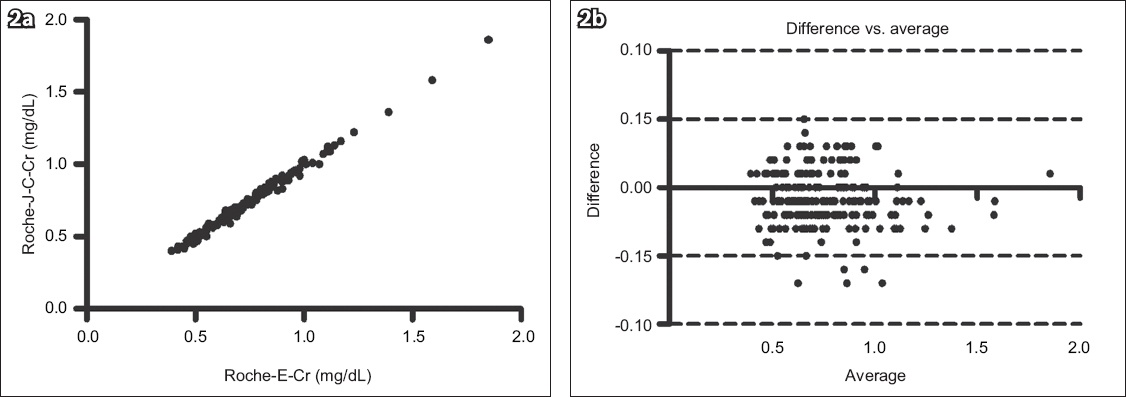
Fig. 3
(a) Scatter graph shows the correlation between the Roche Cobas Integra 400 enzyme creatinine (Roche-E-Cr) and Roche Jaffe creatinine (Roche-J-Cr) methods (n = 163, r2 = 0.9454, p < 0.0001). (b) Bland-Altman plot shows agreement between the two methods (bias = −0.165 ± 0.05).
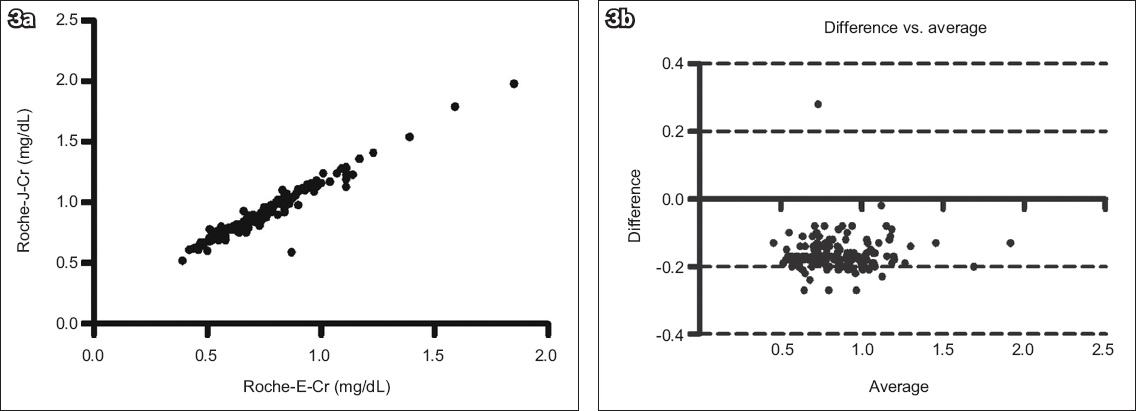
Fig. 4
(a) Scatter graph shows the correlation between the Roche Cobas Integra 400 enzyme creatinine (Roche-E-Cr) and Beckman Jaffe creatinine (Beckman-J-Cr) methods (n = 163, r2 = 0.934, p < 0.0001). (b) Bland-Altman plot shows agreement between the two methods (bias = −0.026 ± 0.07).
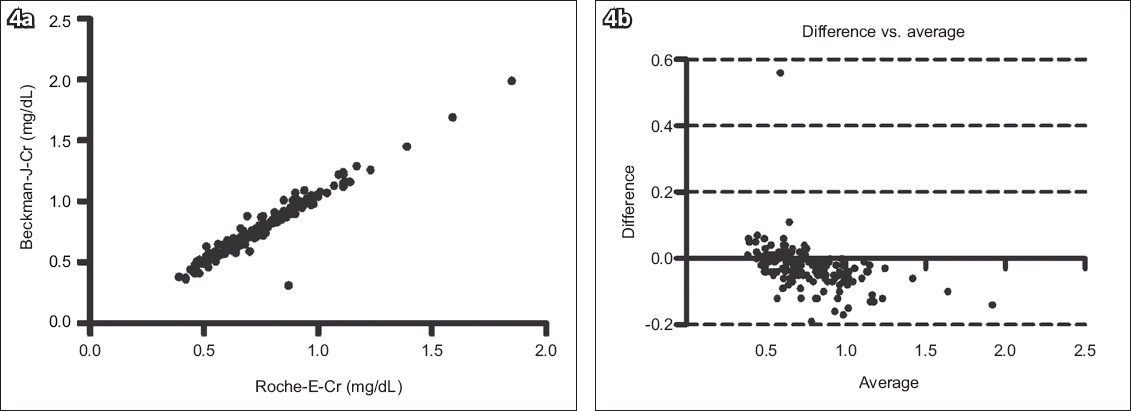
Fig. 5
(a) Scatter graph shows the correlation between the Roche Cobas Integra 400 enzyme creatinine (Roche-E-Cr) and Hitachi Jaffe creatinine (Hitachi-J-Cr) methods (n = 163, r2 = 0.9035, p < 0.0001). (b) Bland-Altman plot shows agreement between the two methods (bias = −0.182 ± 0.08).
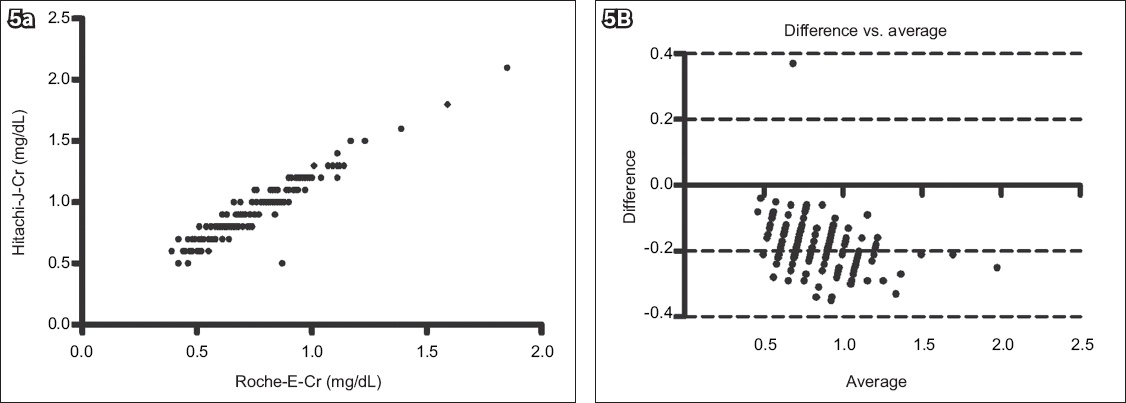
Fig. 6
(a) Scatter graph shows the correlation between the Roche Cobas Integra 400 enzyme creatinine (Roche-E-Cr) and Toshiba Jaffe creatinine (Toshiba-J-Cr) methods (n = 163, r2 = 0.9352, p < 0.0001). (b) Bland-Altman plot shows agreement between the two methods (bias = −0.223 ± 0.06).
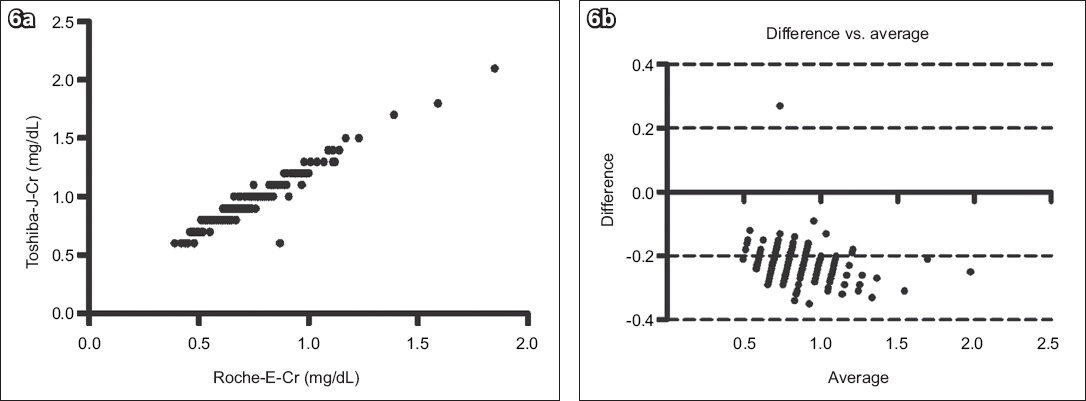
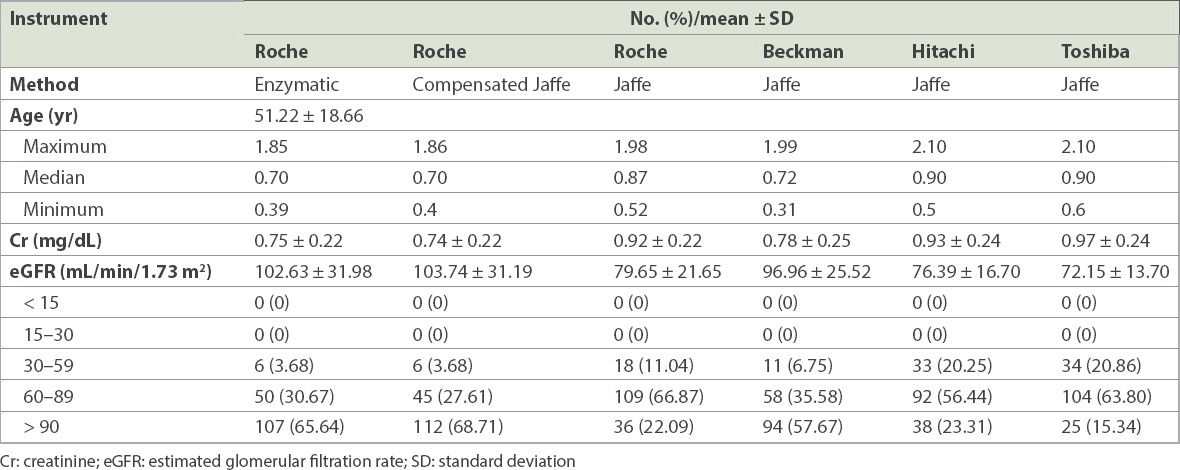
Table II
Baseline characteristics of the participants (n = 163).
DISCUSSION
Although the Scr values in each laboratory were calibrated using IDMS, considerable bias was observed when different equipment was used to measure Scr, affecting the accuracy of the eGFR values. This bias might be related to several factors. First, the calculation method, which traces back to the calibrated IDMS, is a major contributor to the bias. As creatinine calibration in the Jaffe and enzymatic methods merely traced back to the calibrated IDMS, the difference between these two groups should have been limited. However, in our analysis, considerable differences were observed in Scr measurement (Figs.
Second, due to financial constraints and cost-saving policies, some laboratories adjust the parameters of their instruments and use lower-grade reagents and calibration reagents provided by different manufacturers. This variation adversely affects the accuracy of Scr measurement. Previously, during a community screening programme in the Wandan district, Pingtung County, Taiwan, when the Toshiba TBA – c8000 was used to measure 172 Scr samples using the Jaffe reaction, a total of 71 subjects were found with eGFR < 60 mL/min/1.73 m2. However, when the Roche Cobas Integra 400 compensated Jaffe method was used, there were only seven subjects with eGFR < 60 mL/min/1.73 m2. The variation in our result was attributed to differences in reagents and calculations performed by each device.
Third, the NKDEP suggests that the value of Scr measurement should be reported to one decimal place when using the SI unit and to two decimal places when using the regular unit (mg/dL). As shown in
Fig. 7
Bar chart shows the estimated glomerular filtration rate (eGFR) derived using the Roche Cobas Integra 400 Jaffe creatinine (Cr) method. A: Roche Jaffe-Cr (two decimal places); B: Roche Jaffe-Cr (rounded up to one decimal place); C: Roche Jaffe-Cr (rounded down to one decimal place); D: Roche Jaffe-Cr (rounded off to one decimal place).
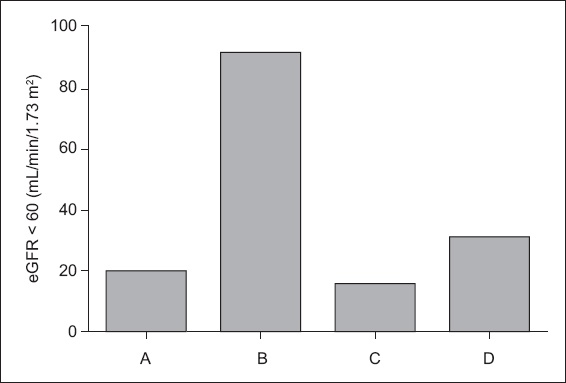
The NKDEP suggests that after calibration of Scr according to IDMS standardisation, the coefficient should be corrected from 186 to 175,(18) and that the uncertainty and analysis bias for Scr must be < 8% and < 5%, respectively.(13) After calibration through IDMS, the difference between the Jaffe and enzymatic methods was minimised (
The Jaffe reaction can be influenced by endogenous chromogens such as acetone, fasting, lipidaemia, haemolysis and antibiotic drugs such as cephalosporins.(17,19) In our study, Scr calibration by the Jaffe reaction was affected by cephalexin (Cefron®). Currently, renal injuries caused by normal dosages of cephalexin have not been documented. However, a greater creatinine reaction is observed when the Jaffe reaction is used, thereby leading to a higher Scr measurement. Therefore, for patients with cephalexin exposure, the enzymatic creatinine method should be used to measure Scr in order to avoid overestimation of Scr level. If the use of the enzymatic method is not feasible, blood Scr levels should be examined before prescription.
In conclusion, various large-scale epidemiological studies have been conducted in Taiwan for determining the incidence and prevalence of CKD. Thus, the accuracy and consistency of inter-laboratory Scr measurements are crucial. Although enzymatic creatinine methods should be used because they are unaffected by nonspecific proteins, haemolysis, vitamin C and drugs, most laboratories select the Jaffe reaction for Scr measurement because the enzymatic methods are expensive. The bias in Scr measurement can be minimised if compensation is performed, the conventional unit mg/dL is used, values are reported up to two decimal places, and the original parameters of the equipment are maintained. We suggest that the Taiwan Society of Nephrology develop a standardised Scr measurement to achieve more effective screening of CKD and conservation of medical resources. CKD is a silent disease that can develop substantially, and standardisation in Scr measurement and GFR estimation is important to facilitate accurate CKD detection.
ACKNOWLEDGEMENTS
We thank the Department of Laboratory Medicine at Antai Medical Care Cooperation, Antai Tian-Sheng Memorial Hospital, Pingtung, Taiwan, for the measurement of serum creatinine. We thank Leader Biomedical Co Ltd for providing reagents. We also thank Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan, and the Department of Prevention and Health Care, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, for their assistance.


