Abstract
INTRODUCTION
Intrauterine insemination (IUI) after controlled ovarian hyperstimulation (COH) was applied to selected infertile patients to determine the effect of gonadotropin-releasing hormone (GnRH) antagonists in IUI cycles, in which recombinant follicle-stimulating hormone (rFSH) had been used for COH.
METHODS
This study was conducted between April 1, 2009 and June 10, 2009, and involved a total of 108 patients. These patients had primary or secondary infertility, which resulted in an indication for IUI, and they each received two cycles of ovarian stimulation treatment with clomiphene citrate. The patients were randomised into two groups – patients in group A received rFSH + GnRH antagonist (n = 45), while those in group B received only rFSH (n = 63).
RESULTS
The mean age of the patients was 31.84 ± 3.73 years and the mean body mass index (BMI) was 24.40 ± 1.88 kg/m2. The mean age and BMI of the patients in groups A and B were not significantly different. There was no significant difference in the mean total rFSH dose administered (988.33 IU in group A and 871.83 IU in group B). When compared to group B, the mean number of follicles that were > 16 mm on the human chorionic gonadotropin (HCG) trigger day was significantly higher in group A (1.58 and 1.86, respectively; p < 0.05). When the two groups were compared, there were no statistically significant differences in the number of cancelled cycles due to premature luteinisation (none in group A vs. two in group B) and the rate of clinical pregnancy (8.9% in group A vs. 7.9% in group B).
CONCLUSION
No significant improvement in the clinical pregnancy rates was observed when GnRH antagonists were used in COH + IUI cycles, despite the significant increase in the number of follicles that were > 16 mm on HCG trigger day.
INTRODUCTION
Intrauterine insemination (IUI) is widely used as an infertility treatment modality. The success of assisted reproductive technologies is dependent on appropriate patient selection and adequate development of oocytes. Compared to clomiphene citrate ovarian stimulation, gonadotropin ovarian stimulation with IUI results in higher pregnancy rates. Moreover, the combination of controlled ovarian hyperstimulation (COH) + IUI has been shown to increase the fecundity of the cycle as compared to IUI alone.(1,2) There are variations in the reported clinical pregnancy rates of COH + IUI cycles; these may be attributed to differences in the aetiology and duration of infertility, sperm preparation technique, the number of sperms injected, the number of inseminations per cycle, cycle monitoring, IUI timing and the ovarian stimulation protocol selected.(2-5)
Although low-dose protocols with recombinant follicle-stimulating hormone (rFSH) are used during COH + IUI cycles,(6) multifollicular development may occur and result in a sudden increase in serum estradiol (E2) levels, which can result in a premature luteinising hormone (LH) peak (before follicular maturation) and revocation of the IUI.(7) Gonadotropin-releasing hormone (GnRH) analogues lead to the desensitisation of pituitary GnRH receptors and, via this phenomenon, block endogenous LH increase. The use of GnRH analogues has been reported to lower premature LH peak to approximately 2% and subsequently increase pregnancy rates.(8) For more than ten years, the use of gonadotropins with GnRH agonists has been the most frequently applied treatment protocol for reducing the incidence of premature LH peak.(9) However, this treatment protocol, which needs 2–3 weeks of desensitisation time, also increases the amount of gonadotropins used, the risk of ovarian hyperstimulation syndrome and the duration of treatment. During the desensitisation period, patients are also exposed to side effects such as hot flushes, headaches, vaginal dryness and bleeding. These drawbacks have resulted in the recent removal of IUI treatment protocols, including GnRH agonists. A Cochrane review on ovarian stimulation protocols for IUI advised against the use of GnRH agonists in COH with low-dose exogenous FSH (i.e. mild COH).(10)
GnRH antagonists, which are produced through the exchange of amino acids of GnRH with other molecules at multiple points, bind to GnRH receptors with high affinity.(11) These antagonistic molecules prevent the release of endogenous gonadotropin and are currently being considered to replace GnRH agonists due to their positive pharmacokinetic and pharmacodynamic properties.(11) The advantages of GnRH antagonists include absence of the first flare-up effect, reduced risk of oestrogen deficiency syndrome (since there is no need for long-term desensitisation), sufficient LH blockage in a short duration of time, dose-dependent effect and fast withdrawal from antagonist effects, which make GnRH antagonists superior to GnRH agonists.(6,12) Furthermore, studies on in vitro fertilisation (IVF) and intracytoplasmic sperm injection protocols have shown that treatment duration and the total dose of gonadotropin used are reduced when antagonists are used in the protocol.(13) It has also been shown that GnRH antagonists are associated with a low risk of high-order multiple pregnancies when standard strict criteria are applied for cycle cancellation.(14)
Although there is controversy regarding the effectiveness of GnRH antagonists in low-dose ovarian hyperstimulation protocols, if current and future studies show that these antagonists are able to improve pregnancy rates, the frequency of their use may increase in the future. In the present study, we studied the effect of GnRH antagonists in IUI cycles, in which rFSH had been used for COH.
METHODS
The present study was conducted between 1 April 2009 and 10 June 2009 in Suleymaniye Maternity and Women’s Disease Research and Teaching Hospital’s outpatient clinics for infertility, the most visited infertility clinics in Istanbul, Turkey. The study was approved by the local ethics committee.
Patients who met the following criteria were eligible for inclusion in the study: (a) indication for treatment with IUI (e.g. unexplained infertility, mild male factor, minimal or mild [stage I or II] endometriosis); (b) a history of two cycles of ovarian stimulation treatment with clomiphene citrate; (c) determination of tubal patency by hysterosalpingography (HSG) or laparoscopy; (d) age 18–39 years; (e) body mass index (BMI) 18–39 kg/m2; (f) regular menstrual cycles (25–32 days); (g) basal FSH < 10 IU/mL, and normal levels of thyroid-stimulating hormone and prolactin; and (h) at least 5 million/mL sperm count and 5% normal morphology on Kruger test. Patients who had clinically significant systemic or endocrine disease, a diagnosis of polyp, submucous myoma, uterine septum or any other space-occupying lesion during HSG or office hysteroscopy evaluation and previous IUI were excluded from the study.
A total of 126 patients agreed to participate in the study and informed consent was obtained. However, only 108 patients were included in the data analysis; 18 patients did not have the appropriate response to treatment, were lost to follow-up or did not have timely sonography. If any one of the following criteria was met, the treatment cycle would be cancelled: (a) premature luteinisation; (b) progesterone level > 1.7 ng/dL during COH on the day of human chorionic gonadotropin (HCG) trigger for ovulation; (c) premature LH peak; (d) LH level > 12.1 mIU/mL on HCG trigger day; (e) probability of multiple gestations due to the presence of more than four follicles > 15 mm; and (f) poor response to treatment (i.e. no follicle > 10 mm).
The patients were randomly divided into two groups (group A and group B) using an online research randomiser software (
When the dominant follicle reached a diameter of > 14 mm, once-daily subcutaneous injection of the GnRH antagonist, Cetrorelix (cetrotide flacon 0.25 mg), was added to the protocol for group A patients; the use of the GnRH antagonist requires continuation of gonadotropin. Cetrorelix was continued until the day of insemination. In both groups, when one, two or three follicles reached a diameter of 17 mm, 10,000 IU HCG (Pregnyl ampoule 5,000 IU, 2 ampoules) was injected intramuscularly, with the aim of triggering ovulation. After a mean duration of 35.5 (range 34–38) hours, IUI was performed. Serum βHCG was measured 14 days after ovulation, and the βHCG test was repeated in patients who tested positive. To determine clinical pregnancy, transvaginal sonography was used to confirm viability at 5–7 weeks of gestation. In both groups, every couple was subjected to only one cycle of treatment. The following efficacy parameters were compared between groups A and B: (a) primary outcome measure – clinical pregnancy rate; and (b) secondary outcome measures – duration of induction, total dose of rFSH used, folliculometry results on HCG day (i.e. number of follicles > 15 mm) and endometrial thickness on HCG day.
Statistical analyses were performed using Number Cruncher Statistical System 2007 and Power Analysis and Sample Size 2008 (NCSS, LCC; Kaysville, UT, USA). Student’s t-test was used for comparing continuous variables that showed normal distribution, while Mann-Whitney U test was used for variables that did not follow a normal distribution. To compare qualitative data, chi-square test and Fisher’s exact test were used. Results were evaluated using 95% confidence interval and p < 0.05 significance level.
RESULTS
A total of 126 patients agreed to participate in the study – 61 patients were assigned to group A and 65 were assigned to group B. In group A, 16 patients were excluded from the data analysis – seven were either lost to follow-up or did not have timely sonography, three had insufficient response to treatment (no development of a dominant follicle), and six patients had cycle cancellation due to > 4 follicles measuring > 15 mm. In group B, one patient was lost to follow-up and one did not develop any follicles. Finally, 45 patients in group A and 63 patients in group B were included in the assessment.
The mean age of the 108 patients included in the final analysis was 31.84 ± 3.73 (range 21–37) years and mean BMI was 24.40 ± 1.88 (range 19–26) kg/m2. The differences in mean age and BMI between groups A and B were not statistically significant.
Table I
Clinical characteristics of the patients (n = 108).
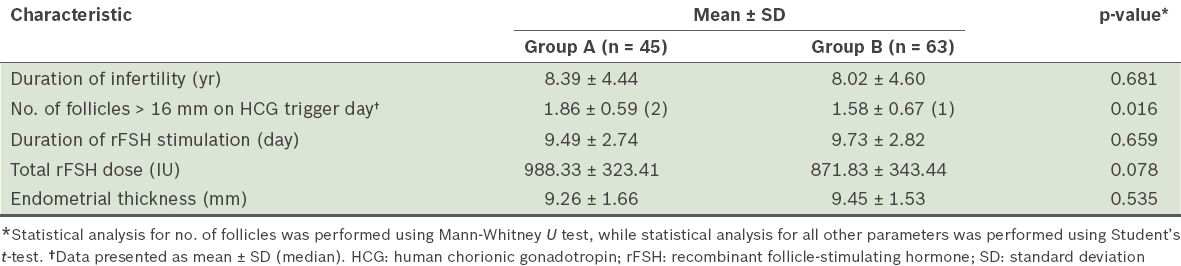
The mean endometrial thickness in group A was 9.26 mm and that in group B was 9.45 mm; however, this difference was not statistically significant. No statistically significant differences were observed in the number of cancelled cycles due to premature luteinisation (none in group A, two in group B) and the rate of clinical pregnancy (8.9% in group A, 7.9% in group B).
DISCUSSION
There is no consensus regarding the use of GnRH antagonists during COH + IUI cycles in the literature. Although earlier investigations have shown the benefits of these molecules,(6,15-18) subsequent studies have failed to confirm them.(19-22) In 2005, Gómez-Palomares et al concluded from their prospective randomised study that the addition of GnRH antagonists to COH + IUI cycles significantly increased pregnancy rates.(15) In 2008, another study by the same authors reported that the markedly better pregnancy rates seen in patients who were administered GnRH antagonists could be due to the multifollicular development of mature (> 18 mm) follicles, as GnRH antagonists allow for the growth of intermediate follicles without the risk of premature luteinisation.(17) Subsequent studies, however, have failed to show the effect of GnRH antagonists on multifollicular development in COH + IUI cycles.(6,19) In a study by Allegra et al, none of the women with an LH level > 10 mIU/mL achieved pregnancy, and the authors concluded that GnRH antagonists improved pregnancy rates by preventing the occurrence of premature luteinisation.(6)
A study by Lambalk et al showed that although GnRH antagonists may reduce the incidence of premature luteinisation, these antagonists do not positively affect pregnancy rate.(16) Crosignani et al’s study also failed to prove that the use of GnRH antagonists is beneficial in IUI cycles; the authors speculated that the benefit of GnRH antagonists in preventing premature LH surge was countered by the unfavourable effects of GnRH antagonists, which were not well understood then.(19) In another study, Martinez-Salazar et al concluded that, while the universal use of GnRH antagonists in COH + IUI cycles does not increase pregnancy rates, it may benefit a specific subset of patients who have premature luteinisation or high progesterone concentrations in a previous failed IUI.(20) It should be noted that Martinez-Salazar et al did not evaluate the LH and progesterone levels during the cycle when GnRH antagonists were used and, therefore, could not determine the exact effect GnRH antagonists had on premature luteinisation.(20) In a multicentre, double-blinded, randomised controlled trial that involved four academic and eight institutional hospitals, Cantineau et al concluded that the addition of GnRH antagonists to standard IUI treatment causes the treatment to be unnecessarily complex, and that GnRH antagonists should thus not be applied in daily practice.(21) In fact, a recent trial evaluating the effectiveness of GnRH antagonists in IUI cycles was discontinued because of the lower pregnancy rates observed in the GnRH antagonist group.(22) Similarly, the 2011 Cochrane review on ovarian stimulation protocols for IUI cycles did not reach a definite conclusion on the use of GnRH antagonists; while the use of GnRH antagonists was not advised in mild COH cycles, it was recommended that the utility of GnRH be determined in future trials.(10)
In the present study, we were not able to prove the benefits of GnRH antagonists in clinical pregnancy rates. However, GnRH antagonists may still be useful in specific infertile subpopulations, such as women with polycystic ovarian syndrome or women in whom COH + IUI cycle has to be converted to an IVF cycle.(23,24) The use of GnRH antagonists has also been shown to be effective in manipulating follicular development, so that the insemination process can be suspended on weekends without an apparent decrease in pregnancy rates.(25,26)
The present study was not without limitations. The number of women enrolled in the study small; nevertheless, other studies investigating the same subject have also enrolled small numbers of patients.(6,16,19) Another limitation was the difference between the number of patients in the two groups due to asymmetric dropouts. This, however, did not affect the significance of the results of statistical analyses performed.
In conclusion, the present study revealed no significant improvement in clinical pregnancy rates when GnRH antagonists were used during COH + IUI cycles, despite a significant increase in the number of follicles > 16 mm on the HCG trigger day. Larger randomised controlled trials are required to determine whether liberal use of GnRH antagonists during IUI cycles should be recommended.


