Abstract
INTRODUCTION
This study was designed and conducted to evaluate the effects of vitamin A, C and E supplementation, and omega-3 fatty acid supplementation on the activity of paraoxonase and arylesterase in an experimental model of diabetes mellitus.
METHODS
A total of 64 male Sprague Dawley® rats, each weighing 250 g, were randomly distributed into four groups: (a) normal control; (b) diabetic control; (c) diabetic with vitamin A, C and E supplementation; and (d) diabetic with omega-3 fatty acid supplementation. The animals were anaesthetised after four weeks of intervention, and paraoxonase and arylesterase activity in blood plasma, and liver and heart homogenates were measured.
RESULTS
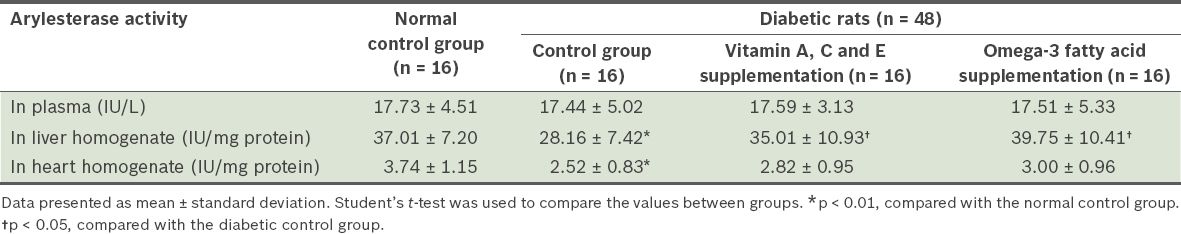
Arylesterase activity in the heart and liver homogenates was significantly lower in the diabetic control group than in the normal control group (p < 0.01). Vitamin A, C and E supplementation, and omega-3 fatty acid supplementation significantly increased liver arylesterase activity (p < 0.05). No significant change was observed in paraoxonase activity and other investigated factors.
CONCLUSION
Vitamin A, C and E, or omega-3 fatty acid supplementation were found to increase liver arylesterase activity in streptozotocin-induced diabetic rats. These supplements may be potential agents for the treatment of diabetes mellitus complications.
INTRODUCTION
Diabetes mellitus, one of the most common metabolic disorders in the world, is known to induce oxidant/antioxidant imbalances. The crucial role of reactive oxygen species in the development and exacerbation of diabetes mellitus complications has been studied for several decades.(1,2) Lipid peroxidation is a degenerative process that affects all lipid-containing structures in cells that are under oxidative stress, leading to cytopathological consequences.(3) Although eukaryotic cells possess primary and secondary defences against the deleterious effects of oxidative stress, serious injury can occur when these defence systems are overwhelmed. In such situations, supplementation with antioxidants such as vitamins A, C and E may be beneficial.(4) Vitamin E, a hydrophobic antioxidant found in lipoproteins and cellular membranes, can provide effective protection against lipid peroxyl radicals.(5) Vitamin C is a hydrophilic antioxidant that can scavenge free radicals; it is likely to act synergistically with vitamin E by reducing oxidised tocopheroxyl radicals back to tocopherol.(6) Vitamin A, on the other hand, plays an important role in modulating insulin response.(7)
Supplementation with omega-3 fatty acids may be useful in preventing diabetes mellitus complications, as long-chain omega-3 fatty acids are incorporated into cell membranes and have anti-inflammatory properties that could prevent diabetes mellitus (omega-3 fatty acids reduce the expression of interleukin-1 beta and human leukocyte antigen class II alleles in activated human monocytes).(8,9) These fatty acids also play a pivotal role in eicosanoid metabolism. There is evidence of abnormal prostaglandin metabolism in children with type 1 diabetes mellitus and anti-inflammatory omega-3 fatty acids (i.e. docosahexaenoic acid and eicosapentaenoic acid) may reduce the risk of disease development.(10) Thus, the present study was designed and performed to evaluate the effects of vitamin A, C and E, or omega-3 fatty acid supplementation on the activity of paraoxonase and arylesterase in liver, heart and blood plasma samples of streptozotocin (STZ)-induced diabetic rats.
METHODS
A total of 64 male Sprague Dawley® rats, aged 15 weeks and weighing 250 g each, were used in this study. During the experimental period, the rats had ad libitum access to standard chow and tap water, and were housed in an environment with a temperature of 18°C–23°C and a 12:12 light-dark cycle. The rats were randomly distributed into four groups of 16 rats each. The four groups were: (a) normal control; (b) diabetic control; (c) diabetic with vitamin A, C and E supplementation; and (d) diabetic with omega-3 fatty acid supplementation.
Diabetes mellitus was induced, via intravascular injection of a single dose of STZ (40 mg/kg body weight), in all animals except those in the normal control group. At one week following the injection, the plasma glucose levels of the rats were checked using the glucose oxidase/p-aminophenazone method of the enzymatic colourimetric test. Rats with a plasma glucose level of more than 300 mg/dL were considered diabetic. All 48 rats that were injected with STZ had a plasma glucose level of more than 300 mg/dL. The diabetic rats in the vitamin A, C and E supplementation group received vitamin A 106 mg/kg, vitamin C 200 mg/kg and vitamin E 250 mg/kg daily by oral gavage, while diabetic rats in the omega-3 supplementation group received omega-3 fatty acids 300 mg/kg daily by oral gavage.
After four weeks of intervention, the rats were anaesthetised and arterial blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA). After the blood samples were centrifuged at 2500 revolutions per minute (rpm) for five minutes, the plasma was separated and the erythrocytes were washed three times in 5 mL of 9% sodium chloride (NaCl). Total haemoglobin was measured and the erythrocytes were stored at –70°C until analysis for biochemical factors. Liver and heart tissues were promptly excised from anaesthetised rats and washed with normal saline (NaCl 0.9%). The harvested tissues were then dried using filter paper and stored at –70°C.
To determine catalase and superoxide dismutase activities, 1:11 (w/v) tissue homogenate was prepared in potassium phosphate buffer (pH 7.4) 50 mM, potassium chloride 150 mM and EDTA 200 mM. The mixture was homogenised using a Potter-Elvehjem Tissue Grinder and centrifuged at 15,000 rpm for 30 minutes. The protein concentration of the homogenate was then determined using the biuret protein assay, with bovine serum albumin as the protein standard.(11) Paraoxonase activity in the liver and heart homogenates, and blood plasma was measured using the rate of paraoxon hydrolysis at 412 nm. As most commercial paraoxon samples contain breakdown products, some of which interfere with the spectrophotometric assay of paraoxonase, it was necessary to purify the paraoxonase prior to use.(12) Arylesterase activity in the liver and heart homogenates, and blood plasma was measured using the rate of phenylacetate hydrolysis at 270 nm.
Data was presented as mean ± standard deviation. SPSS version 11.5 (SPSS Inc, Chicago, IL, USA) was used for all analyses. Independent sample t-test was used to compare the mean between groups. A value of p < 0.05 was considered to be statistically significant.
RESULTS
The levels of paraoxonase and arylesterase activity in the different experimental groups are presented in Tables
Table I
Paraoxonase activity in the plasma, and heart and liver homogenates of the four groups of rats.
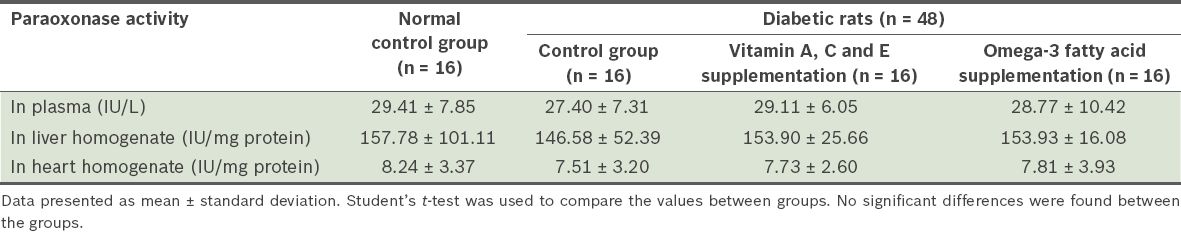
Table II
Arylesterase activity in the plasma, and heart and liver homogenates of the four groups of rats.
DISCUSSION
Based on the data obtained in the present study, which involved rats with STZ-induced diabetes mellitus, arylesterase activity was lower in the heart and liver homogenates of diabetic control rats as compared to that of rats from the normal control group (p < 0.01). The results of the present study also showed that after four weeks of vitamin A, C and E supplementation, or omega-3 fatty acid supplementation, arylesterase activity was enhanced in the liver homogenates of the diabetic rats (p < 0.05). The level of paraoxonase activity was not found to be significantly different after supplementation with vitamins A, C and E, or omega-3 fatty acids.
Paraoxonase, which has been shown to decrease in diabetic subjects, may be a predictor of complications of diabetes mellitus.(13) Both Kang et al and Sampson et al reported a decrease in paraoxonase activity in patients with diabetes mellitus.(14,15) This may be due to the inactivation of paraoxonase via glycation, a decrease in its gene expression or the inhibition of high-density lipoprotein synthesis or secretion, which is reported to be associated with serum paraoxonase.(14,16) In the present study, we did not observe any significant changes in paraoxonase activity. A possible reason is the relatively short intervention period of four weeks, which may not have been sufficient to detect the effect of supplementation. This is one of the limitations of our study.
It has also been shown that glucose autoxidation, protein glycation and the production of advanced glycation end products are the main mechanisms by which oxygen free radicals are produced in patients with diabetes mellitus.(17,18) In diabetic patients, beta-oxidation of fatty acids increases as a result of insufficient insulin. This, in turn, results in the accumulation of hydrogen peroxide in tissues, leading to enzyme inactivation via glycation. Due to the higher concentration of hydrogen peroxide in the tissue than in the blood, enzyme inactivation via glycation is more pronounced in the tissues.(19)
The liver is the main organ responsible for synthesising paraoxonase and arylesterase; thus, inhibiting oxidative damage with antioxidant (i.e. vitamins A, C and E, and omega-3 fatty acids) supplementation causes significant changes in the activity of these two key enzymes in the liver homogenate. As vitamins A, C and E have different sites of action and different mechanisms to prevent oxidative damage, these vitamins are expected to be more effective when used in combination than individually.(20) The ability of ascorbic acid (vitamin C) to reduce alpha-tocopheroxyl radicals to alpha-tocopherol, inhibiting the resulting oxidative damage, has been demonstrated in both in vitro and in vivo studies.(21,22) Vitamin E may help regulate intracellular magnesium levels, which diminish with increased oxygen free radical production. While vitamin C scavenges reactive oxygen species in the hydrophilic milieu, alpha-tocopherol and vitamin A inhibit lipid oxidation by free radical chain reaction in the hydrophobic domains of the bilayer.(23,24) Supplementation with vitamins C and E has been reported to increase antioxidant enzyme activity.(25) Similarly, omega-3 fatty acid supplementation might also increase antioxidant enzyme activity.(26) Although the underlying mechanism responsible for the beneficial effects of omega-3 fatty acids on oxidative status has not been fully understood, several studies indicate that omega-3 fatty acids may play a pivotal role in decreasing the complications of diabetes mellitus.(27,28)
In conclusion, the results of the present study indicated that four weeks of supplementation with vitamins A, C and E, or omega-3 fatty acid can improve the activity of arylesterase in the liver. It may do this by decreasing free radical production and/or inhibiting enzyme destruction. Further study is needed to shed light on the specific mechanisms by which such supplementation improves antioxidant enzyme activity.
ACKNOWLEDGEMENT
This study was conducted using a grant from the Tehran University of Medical Sciences, Iran.


