Abstract
INTRODUCTION
Endoscopic submucosal dissection (ESD) in the colon and rectum has been developed with good reported outcomes. The main advantage of ESD is the ability to perform en bloc resection, which has implications for complete excision and pathological analysis. Locally, the use of ESD in colonic lesions has seen recent traction. Our study aimed to review the outcomes of the first 50 cases of endoscopic excision of advanced colonic lesions using ESD at our institution.
METHODS
This was a retrospective study of all patients who underwent ESD at our institution from September 2010 to October 2016. Data collected included patient demographics, resection technique, conversion rate and morbidity.
RESULTS
51 patients underwent ESD during the study period. All patients were of American Society of Anesthesiologists (ASA) class 1–3. Their median age was 60.0 years and the majority (n = 36) were male. The mean procedure time was 80.9 minutes. 36 (76.6%) of cases underwent en bloc resection. 4 (7.8%) cases required conversion to surgery, mainly due to difficulty in raising the colonic lesions. 3 (5.9%) patients had malignancy as the final histology. 2 (4.3%) patients had recurrence during surveillance scope. No cases of early mortality were reported.
CONCLUSION
Our results suggest that ESD for advanced colonic lesions can be safely performed. Expertise needs to be developed to achieve satisfactory en bloc resection rates.
INTRODUCTION
The link between colorectal carcinoma (CRC) and adenoma is now well established.(1) As such, the complete removal of these adenomas reduces the risk of developing CRC. Studies have shown that one of the main contributing factors for local recurrence is the incomplete resection of lesions.(2) Large and sessile adenomas remain a challenge for complete excision and patients may undergo surgical resection instead. The development of endoscopic resection provided a less invasive option of removing lesions that cannot be snared by conventional methods. In particular, endoscopic mucosal resection (EMR) has emerged as a treatment for flat and sessile colonic polyps.(3) However, EMR is not ideal for larger lesions, as the possibility of piecemeal resection is higher. This has implications for the completeness of the resection, pathological analysis and recurrence rates, thus affecting management and follow-up surveillance plans.(4)
Endoscopic submucosal dissection (ESD) was first described in the ‘90s by the Japanese in the context of gastric tumour removal. Aiming to achieve en bloc resection and lower local recurrence rates, endoscopists started applying this technique to colonic polyps.(5) The five-year cancer recurrence rate following ESD was reported at 1.6%.(6) Despite the advantages of ESD, EMR remains the more common technique in Western countries due to several reasons, one being the lack of training opportunities.(7) Furthermore, ESD is technically more demanding and time-consuming due to the higher complication rates compared to EMR.(8) Given the usefulness of ESD, this paper aimed to present our institution’s experience of its first 50 cases of endoscopic resection of advanced colonic lesions using ESD.
METHODS
We reviewed the data of 51 patients who underwent endoscopic resection of colonic lesions using ESD. These resections were performed by a single endoscopist from September 2010 to October 2016. Patients who had polyps with the following characteristics were selected for endoscopic resection: broad-based, flat, and with pit or vascular patterns on magnified views suggestive of adenoma. These polyps were deemed not suitable for conventional snaring. Patient factors analysed were age, gender, American Society of Anesthesiologists (ASA) physical classification, resection techniques, types of resection, size and location of lesions, histology of lesions, rate of conversion to surgery and complication rates (
Table I
Baseline characteristics of patients (n = 51).
Lesions that were not considered for endoscopic resection were those with strong suspicion of massive submucosal involvement. Such endoscopic features include ulceration and white coating, spontaneous bleeding, mucosal fold convergence and suspicious pit or vascular patterns on magnified views. As not all referring endoscopists were trained in identifying these features, this exclusion was not absolute. Biopsies were not performed in cases prior to endoscopic excision if image enhancement showed features typical of adenoma.
All ESD procedures were performed under sedation. Equipment used was: flexible endoscopes with a distal hood, the DualKnife™ electrosurgical knife (Olympus, Tokyo, Japan) and the Coagrasper™ Haemostatic Forceps (Olympus, Tokyo, Japan). Colonic lesions were raised using hyaluronic acid eye drops with indigo carmine. An incision of the mucosal layer was made using a DualKnife, followed by an incision of the submucosal plane using the same knife. Haemostasis of the submucosal plan was performed through direct coagulation with the DualKnife or with the haemostatic forceps. The resection was completed in some lesions using the Captivator™ II Single Use Snare (Boston Scientific, Natick, MA, USA) after adequate mucosal incision and submucosal dissection (Figs.
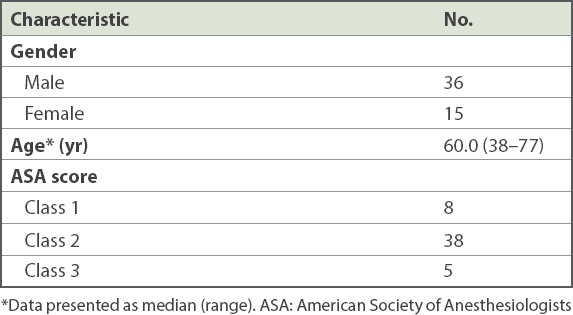
Fig. 1
Photographs shows broad-based transverse colon lesions in (a) initial endoscopic view and (b) prior to commencement of submucosal dissection.
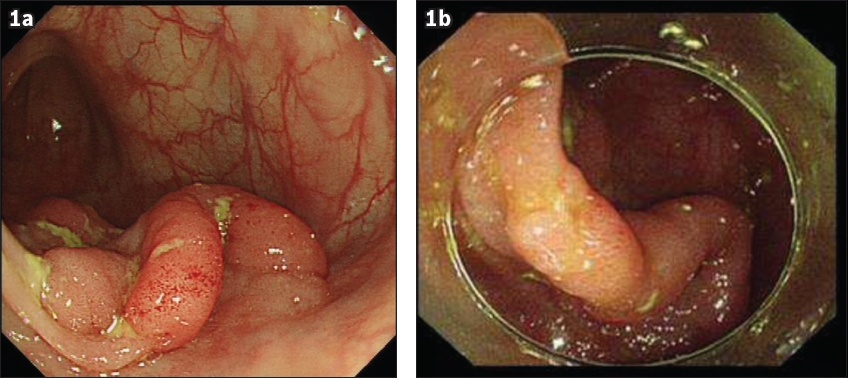
Fig. 2
Photographs show (a) the process of submucosal dissection and (b) the completed submucosal resection.
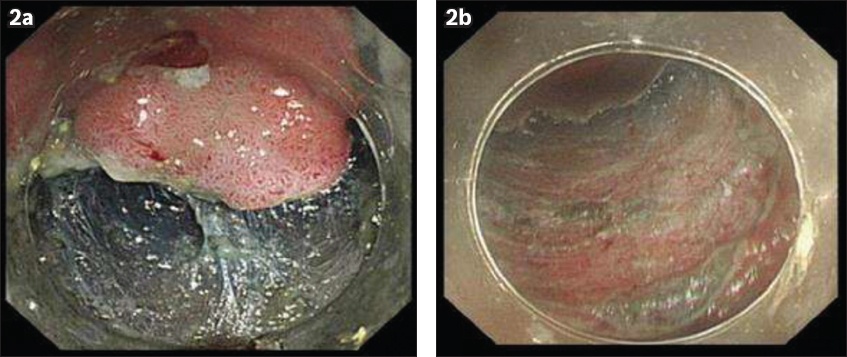
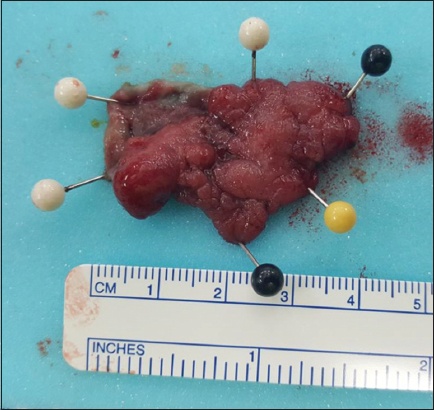
All specimens were pinned to demonstrate en bloc excision (
Fig. 3
Photograph shows an en bloc excision specimen pinned out for histology.
In terms of post-procedure care, all patients were started on either a liquid or full diet. No routine blood tests or imaging were performed after the procedure. The majority of the patients were discharged on post-procedure Day 1 if there was no clinical evidence of complications such as bleeding and perforation.
RESULTS
The mean duration of our ESD procedures was 80.9 (range 21–300) minutes. The excised lesions had a median size of 2.00 cm and a mean size of 1.93 (range 0.5–4.5) cm. A longer procedure time was recorded for larger lesions (
Table II
Endoscopic submucosal dissection results based on size of lesions.
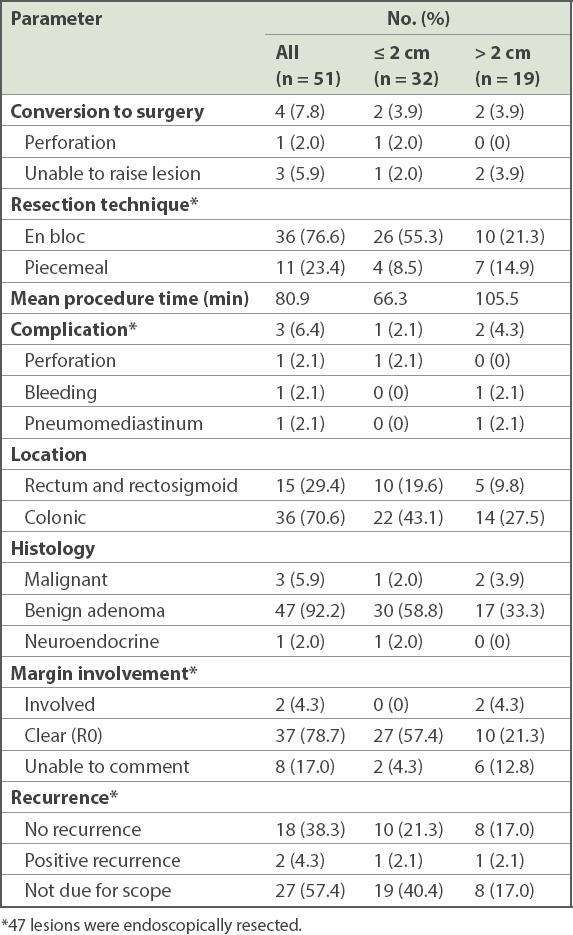
On surveillance colonoscopy, 2 (4.3%) patients had recurrence. Repeated excision showed that both lesions were non-malignant. In both of these cases, the ESD had been performed with piecemeal technique during the initial procedure due to the large size of the lesions, and margins were histologically involved during the first resection. 27 (57.4%) patients had not yet undergone surveillance colonoscopy at the time of the study.
There were 3 (6.4%) cases with complications from ESD. One of the patients developed pneumomediastinum, with a small pneumothorax that resolved with conservative management. Another patient had bleeding less than 24 hours after the procedure and required a repeat colonoscopy for haemostasis. The third case was converted to surgery due to a pinpoint perforation during ESD. Surgery was performed rather than attempting to close the defect, as there was difficulty raising the lesion and a suspicion of underlying malignancy. No mortality within 30 days following ESD has been reported in our institution thus far.
DISCUSSION
EMR was the standard for non-invasive treatment of colonic lesions before the introduction of ESD.(3,5) However, various recent studies have demonstrated that ESD is becoming the preferred practice for larger lesions. ESD provides better R0 resection rates compared to EMR without exposing patients to the higher risks of surgery (laparoscopic or open resection).(8) This study demonstrated the effectiveness of ESD for large colonic lesions.
Research by surgeons in Korea has described highly promising R0 and en bloc resection rates of 90.5% and 97.1%, respectively.(9) Another study conducted in a single European centre reported R0 and en bloc resection rates of 62% and 88%, respectively, with ESD, which is more consistent with our data.(8) There is no doubt that our results are inferior to those of Japan and Korea, showing the presence of a learning curve and the need for continued improvement of our techniques. Local challenges include the need for our endoscopists to have a more broad-based practice rather than subspecialise as pure endoscopists. A systemic review by Repici et al that examined 13 papers found a local recurrence rate of 0.07% for cases with R0 resection during a median of two years’ follow-up.(10) Studies done by the Japanese found five-year local recurrence rates of approximately 1.5%.(6,11) Based on our study, recurrence was associated with piecemeal resection. Indeed, piecemeal resection has been observed to be the most influential factor in local recurrence for both ESD and EMR.(12) Surveillance colonoscopy has been recommended one year after en bloc resection, while interval follow-up of six months is recommended for piecemeal resection.(6)
The long procedure time is one of the main drawbacks for ESD compared to EMR or polypectomy. A study conducted by a Korean group recorded a median time of 53 minutes for ESD, whereas another study by a Western country had a median time of 105 minutes.(13,14) The present study had median time of 80.9 minutes. Our results also showed that larger lesions required a longer procedure time.
Our study had three patients with adenocarcinoma found in endoscopically resected specimens. In these cases, the decision for definitive surgical resection depends on the pathological features of the carcinoma. Lesions with deep submucosal invasion, tumour budding, high-grade tumour differentiation and lymphovascular invasion would have a higher risk of lymph node metastasis and thus mandate surgical resection.(15,16) The need for such pathological evaluation also emphasises the value of en bloc resection of these lesions.
A meta-analysis by Akintoye et al reported perforation and bleeding as the most common post-ESD complications.(17) Another meta-analysis by Fujiya et al showed higher rates of complications following ESD compared to EMR (5.7% vs. 1.4%).(18) The higher complication rate is one of the main reasons ESD is less commonly used in Western countries. In another study, rates of intra-procedure perforation for ESD were 4.5%, while risk of bleeding was 1.3%.(5) Our study recorded 1 (2.1%) patient with perforation during ESD who required immediate conversion to surgery. Despite the higher risk of perforation with ESD, some studies have argued that most perforations can be treated with endoscopic clipping, while conservative management with oral antibiotics can be used for delayed micro-perforations.(19) Hotta et al stated that one criteria for conservative management of post-ESD perforation is the absence of peritonism.(20)
There were several limitations to our study. First, it was a retrospective study performed at a single institution and had a single-operator design. Additionally, not all patients who underwent ESD were followed up, as 52.9% of them were not due for surveillance colonoscopy. There has been a steady increase in the number of ESD procedures performed in our institution over time, which is likely secondary to referral patterns as well as increased awareness and trust in the technique of endoscopic resection. Correspondingly, fewer adenoma cases underwent surgical resection. According to Gotoda et al, proficiency in ESD requires experience of at least 30 cases.(21) Hence, our experience in ESD is extremely basic compared to countries such as Japan. It is evident that we started with smaller lesions in the more distal colon and subsequently progressed to larger, more proximal lesions. A larger number of samples and longer period of time are needed to assess the long-term outcome of ESD at our institution. This report marks our observations and results upon reaching a milestone of 50 cases.
In conclusion, endoscopic resection of advanced colonic lesions using ESD is feasible but requires specific expertise. This capability needs to be further developed, as the technique can allow a greater possibility of R0 excisions and is ultimately less invasive than surgical resection.


