Abstract
INTRODUCTION
This study aimed to evaluate the potential of non-contrast-enhanced magnetic resonance (MR) imaging as an imaging surveillance tool for detection of hepatocellular carcinoma (HCC) in at-risk patients and to compare the performance of non-contrast MR imaging with ultrasonography (US) as a screening modality for the same.
METHODS
In this retrospective study, patients diagnosed with HCC between 1 January 2010 and 31 December 2015 were selected from our institution’s cancer registry. Patients who underwent MR imaging and had US performed within three months of the MR imaging were included. For each MR imaging, two non-contrast MR imaging sequences – T2-weighted fat-saturated (T2-W FS) sequence and diffusion-weighted imaging (DWI) – were reviewed for the presence of suspicious lesions. A non-contrast MR image was considered positive if the lesion was seen on both sequences. The performance of non-contrast MR imaging was compared to that of hepatobiliary US for the detection of HCC.
RESULTS
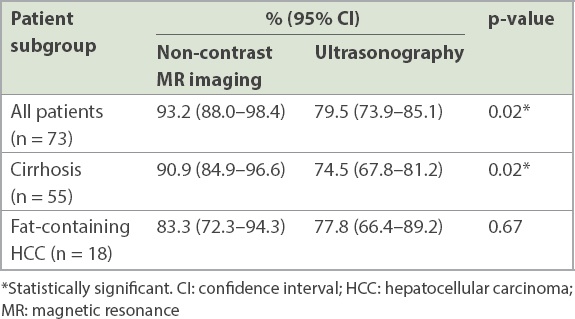
A total of 73 patients with 108 HCCs were evaluated. Sensitivity of non-contrast MR imaging for the detection of HCC using T2-W FS and DWI was 93.2%, which was significantly higher than that of US, which was 79.5% (p = 0.02). In a subgroup of 55 patients with imaging features of liver cirrhosis, the sensitivity of non-contrast MR imaging was 90.9%, which was also significantly higher than that of US, which was 74.5% (p = 0.02).
CONCLUSION
Our pilot study showed that non-contrast MR imaging, using a combination of T2-W FS and DWI, is a potential alternative to US as a screening tool for surveillance of patients at risk for HCC.
INTRODUCTION
Ultrasonography (US) is currently the recommended imaging screening test for patients at risk of hepatocellular carcinoma (HCC).(1,2) Established guidelines recommend six-monthly US examinations for these patients, and nodules greater than 1 cm are further evaluated using multiphasic computed tomography (CT) or magnetic resonance (MR) imaging.(1)
US has a variable sensitivity range of 58%–89% and a specificity of over 90%.(3,4) Factors such as operator experience and patient habitus affect the quality of US, which limits its sensitivity. Moreover, in cirrhotic livers, generalised coarsened and nodular appearance of the parenchyma make it challenging for the operator to detect focal lesions.(1,5) One meta-analysis that pooled data comparing US, CT and MR imaging concluded that US has lower sensitivity than CT or MR imaging for the detection of HCC.(6) However, CT is not feasible for screening owing to radiation concerns and the mandatory need for intravenous contrast, which has been associated with acute kidney injury and can be contraindicated for some individuals.
The use of limited non-contrast and contrast MR imaging as a screening tool has recently been explored. Han et al evaluated the diagnostic performance of four non-contrast MR imaging sequences, T2-weighted single-shot fast-spin echo, T2-weighted with fat-saturation (T2-W FS) sequence, T1 gradient in- and out-of-phase sequences, and diffusion-weighted imaging (DWI), and concluded that it was a potential surveillance tool for detecting HCC.(7) Hecht et al suggested that contrast MR imaging protocol using only T1-weighted (T1-W) pre-contrast and dynamic post-contrast sequences was comparable to a full MR imaging study, which includes T2-W FS and DWI sequences.(8) One study showed MR imaging with hepatocyte-specific contrast agents to have a higher sensitivity of 70% for the detection of early-stage HCC when compared to US (63%).(4) However, intravenous gadolinium contrast may not be acceptable for large-scale screening owing to current controversies regarding gadolinium deposition in tissues as well as the costs involved.(9) Non-contrast MR imaging is a potential alternative to US as a screening modality. However, established data from large-scale studies on non-contrast MR imaging is lacking and needs to be further evaluated for ascertaining its feasibility as a surveillance modality.(10)
In this retrospective study, we aimed to evaluate the feasibility of an abbreviated non-contrast MR imaging protocol utilising two sequences, T2-W FS and DWI, as a screening tool for patients at risk of HCC.
METHODS
This was an institutional review board-approved retrospective study performed at Tan Tock Seng Hospital, Singapore, with waivers of informed consent from the participants. A list of patients with HCC diagnosed at our institution from 1 January 2010 to 31 December 2015 was obtained from our institution’s cancer registry. These patients were selected via random sampling to reduce selection bias. Inclusion criteria were: (a) HCC confirmed by histological analysis or fulfilling imaging criteria; (b) contrast-enhanced MR imaging of the liver at diagnosis and being deemed at risk for HCC (chronic viral hepatitis or cirrhosis); and (c) recent hepatobiliary US imaging, defined as imaging performed within three months of MR imaging. Exclusion criteria were: (a) MR imaging performed without intravenous contrast; (b) suboptimal T2-W FS or DWI sequences due to artefact or scan factors; and (c) having another known primary malignancy at the time of HCC diagnosis.
For each patient, MR images were retrieved electronically from the institution’s Picture Archiving and Communication System. As most patients had multiple MR images, only the imaging at the time of diagnosis was reviewed. The electronic medical records were also reviewed. Patient demographics, alpha-fetoprotein levels, hepatitis status and MR imaging findings at the time of diagnosis were recorded. Sample size was calculated based on power analysis.
MR imaging studies were performed using either a 1.5 tesla (1.5T) MR scanner (Signa™; GE Medical Systems, Milwaukee, WI, USA) or a 3.0 tesla (3T) MR scanner (Magnetom Trio™; Siemens Healthcare, Erlangen, Germany), with body phased-array receiver coil (either an eight-element or six-element phased-array body matrix surface coil for the 1.5T scanner or 3T scanner, respectively) and integrated spine-array receiver coil embedded into the scan table (either an eight-element or 24-element spine matrix coil for the 1.5T or 3T scanner, respectively).
T2-W FS breath hold or respiratory-triggered sequences were acquired through the whole liver in the axial plane with the following parameters: slice thickness 4–8 mm; slice spacing 7–8 mm; echo time 77–113 ms; repetition time 4,800–8,500 ms; flip angle 90°; and matrix size of 320–512 × 240–512.
Axial fat-saturated single-shot echo planar DWI sequences were acquired with the following parameters: slice thickness 4–8 mm; slice spacing 7–8 mm with b-values of 0 second/mm2, 500 second/mm2 and 1,000 second/mm2; echo time 66–76 ms; repetition time 3,850–6,250 ms; flip angle 90°; and matrix size of 128–256 × 128–256.
Other standard MR imaging sequences that were acquired but not specifically evaluated in our study were: coronal T2-W single-shot fast-spin echo; axial T1-W in- and out-of-phase sequences; and multiphasic contrast-enhanced three-dimensional (3D) T1-W sequences in the axial plane using either gadoterate meglumine (Dotarem®; Guerbet, France), gadobenate dimeglumine (MultiHance®; Bracco, Milan, Italy) or gadoxetate disodium (Primovist®; Bayer, Germany) as an intravenous contrast agent.
Images were read, in consensus, by two radiologists: a final-year (i.e. Year 5) radiology resident-in-training and a board-certified abdominal radiologist with seven years of experience in reading liver MR images.
In the first reading session, only the T2-W FS and DWI sequences for each patient’s MR image were reviewed. The T2-W FS sequence was considered positive if there was a focal lesion of intermediate hyperintense signal similar to that of the spleen (
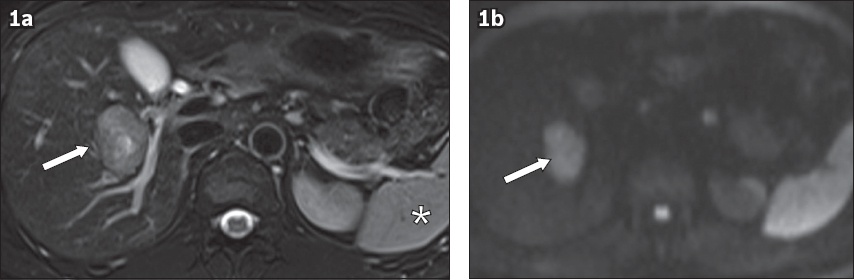
Fig. 1
(a) Axial T2-W fat-saturated sequence (FS) and (b) diffusion-weighted imaging (DWI) sequences show typical imaging findings of a hepatocellular carcinoma lesion. (a) T2-W FS sequence shows a 3.7-cm lesion of intermediate heterogeneous hyperintense signal in the right hepatic lobe (arrow), adjacent to the right hepatic vein, having similar signal intensity to the spleen (asterisk). (b) DWI sequence shows the lesion demonstrating restricted diffusion at a b-value of 1,000 s/mm2, appearing hyperintense (arrow).
In the second reading session conducted about a week after the first reading session, the post-contrast MR imaging sequences and histological results were reviewed. This was correlated with findings from the first reading session to determine the site and size of HCC for each patient.
In the third reading session conducted one week after the second reading session, the hepatobiliary US scans performed within three months of MR imaging were reviewed to determine if the HCC could be visualised on US. A US scan was considered positive if the focal lesion corresponded to the HCC seen on contrast-enhanced MR imaging.
The readers were blinded to the findings on contrast-enhanced MR imaging when reviewing the non-contrast MR imaging sequences and US scans.
In our study, the diagnosis of HCC was made on either histological examination (biopsy or surgical resection), or if the lesion fulfilled typical enhancement characteristics on CT or MR imaging of arterial phase hyperenhancement and washout appearance for at-risk patients.(12)
Sample size was estimated by a power analysis using a two-sample, two-sided equality test (a = 0.05, b = 0.8), assuming 90% sensitivity for non-contrast liver MR imaging and 60% sensitivity for US for detection of HCC.(4,13) Therefore, a sample size of at least 62 patients was required for the study.
Owing to our methodology of selecting patients, all included patients had a confirmed diagnosis of HCC (positive reference standard) without a case-control group (negative reference standard). Therefore, only point estimates for sensitivity of non-contrast MR imaging and US could be derived, using the formula Sn = TP/(TP + FN), where Sn was sensitivity, TP was true positive and FN was false negative.
Sensitivity of non-contrast MR imaging was compared to that of US using the McNemar chi-square test for paired observations. Significance level for all comparisons was set at 5%. Statistical analysis was performed using IBM SPSS Statistics for Windows version 21.0 (IBM Corp, Armonk, NY, USA).
RESULTS
A total of 73 patients (108 HCCs) were included (61 male, 12 female). The mean patient age was 71.9 (range 47–92) years. Overall, 54 patients had serological confirmation of chronic hepatitis B while 55 patients had imaging features of cirrhosis. There were 35 HCCs with size < 2 cm, 22 HCCs ranging 2–3 cm and 51 HCCs > 3 cm.
For the per-lesion analysis, 96 HCCs were detected on both T2-W FS and DWI, four HCCs were not detected on T2-W FS but detected on DWI, one HCC was detected on T2-W FS but not detected on DWI, and seven HCCs were not detected on either of the modalities. This yielded a sensitivity of 88.9% (95% confidence interval [CI] 84.5%–93.3%) for non-contrast MR imaging for the detection of HCC. A total of 55 patients had imaging features of liver cirrhosis (with 84 HCCs). In this group of patients, there were 72 HCCs with a positive non-contrast MR study, yielding a sensitivity of 85.7% (95% CI 80.7%–90.7%) for non-contrast MR imaging for the detection of HCC.
For the per-patient analysis of US for the detection of HCC, 58 of 73 patients had at least one HCC visualised, yielding a sensitivity of 79.5% (95% CI 73.9%–85.1%). Among 68 patients, at least one HCC was seen on both T2-W FS and DWI sequences (positive non-contrast MR imaging), yielding a sensitivity of 93.2% (95% CI 88.0%–98.4%). In this group of patients, the sensitivity of non-contrast MR imaging was significantly higher than that of US (p = 0.02).
Three patients (with four HCCs) had a positive US but negative non-contrast MR imaging. The MR imaging features of the liver and HCCs in these three patients were further evaluated. In two patients, MR imaging or US features of liver cirrhosis were observed (
Fig. 2
(a) Axial T2-W fat-saturated (FS) and (b) diffusion-weighted imaging (DWI) sequences show cirrhotic liver obscuring a hepatocellular carcinoma (HCC) lesion on abbreviated non-contrast MR imaging. Note the diffuse heterogeneous appearance of the liver on T2-W FS due to cirrhosis, which obscured the HCC in the right liver lobe. (c) Post-contrast T1-W arterial phase MR image shows partial enhancement of the HCC in the right lobe (arrow). (d) Post-contrast axial T1-W hepatobiliary phase MR image clearly shows the HCC as hypointense to the adjacent liver parenchyma (arrow).
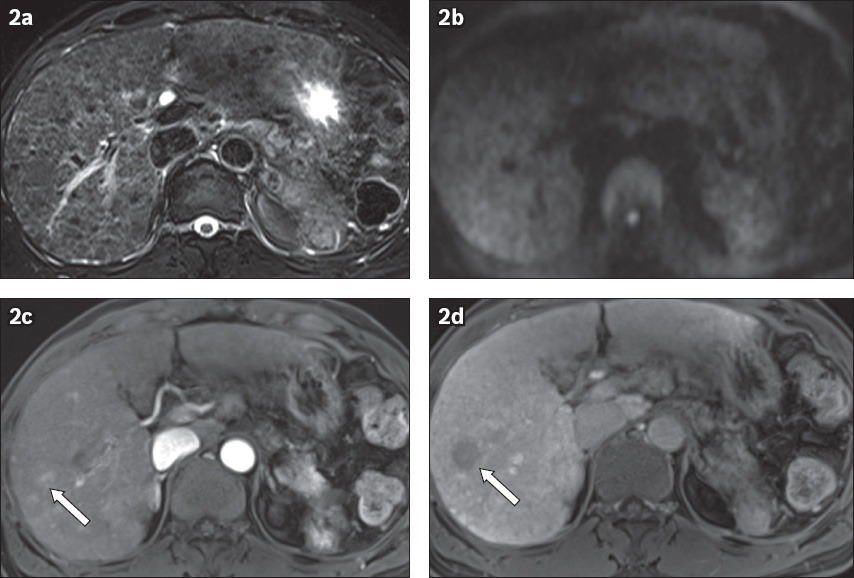
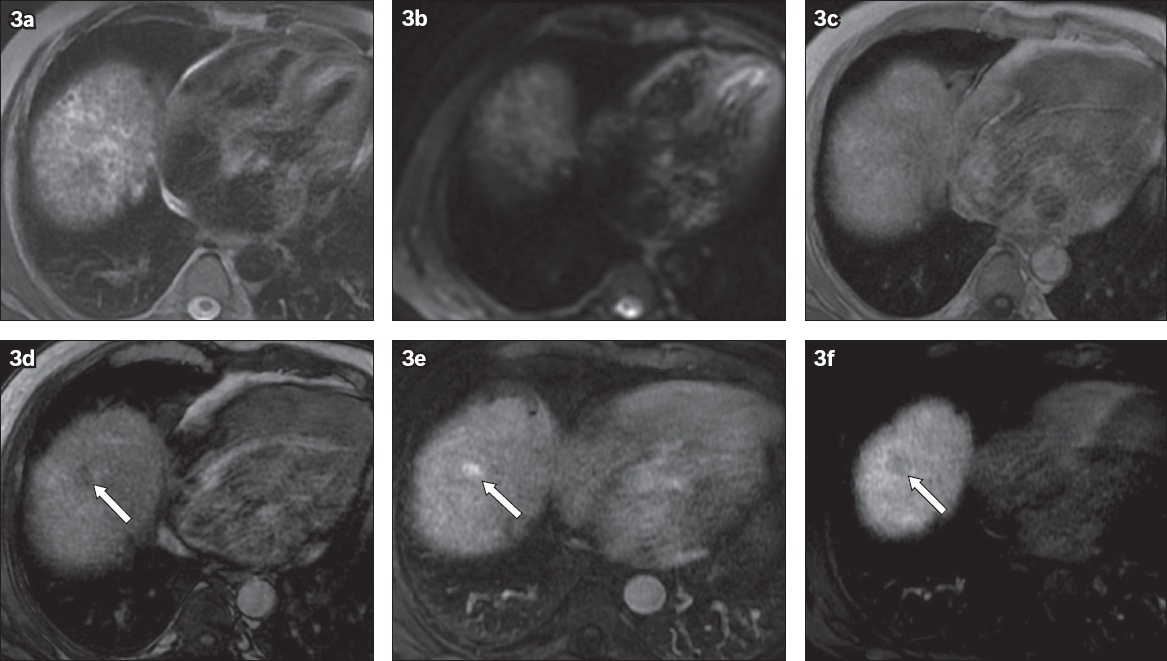
Fig. 3
Fat-containing hepatocellular carcinoma was not seen on non-contrast MR imaging: (a) non-contrast axial T2-W fat-saturated and (b) diffusion-weighted imaging sequences show no visible lesion. Note the background heterogeneous appearance of the liver secondary to cirrhosis. (c) In-phase and (d) out-of-phase sequences show a fat-containing lesion in hepatic segment VIII, which appeared as a hypointense lesion on the out-of-phase sequence (arrow). (e) Post-contrast arterial phase and (f) 180-second transition phase sequences show arterial enhancement with washout (arrow).
Among 55 patients with imaging features of liver cirrhosis, 41 patients had at least one HCC visualised on US, yielding a sensitivity of 74.5% (95% CI 67.8%–81.2%). Meanwhile, 50 patients had at least one HCC visualised on non-contrast MR imaging, yielding a sensitivity of 90.9% (95% CI 84.9%–96.6%). In this group of patients with cirrhosis, the sensitivity of non-contrast MR imaging was also significantly higher than that of US (p = 0.02).
Among 18 patients with fat-containing HCCs, 14 patients had at least one HCC visualised on US, yielding a sensitivity of 77.8% (95% CI 66.4%–89.2%), while 15 patients had at least one HCC visualised on non-contrast MR imaging, yielding a sensitivity of 83.3% (95% CI 72.3%–94.3%). In this group of patients with fat-containing HCC, no significant difference was observed in the sensitivities of non-contrast MR imaging and US (p = 0.67).
The results of the overall and subgroup analysis are summarised in
Table I
Sensitivity of non-contrast MR imaging compared to ultrasonography for screening of patients at risk of HCC.
In our patient cohort, there were no foci that were positive on both T2-W FS sequence and DWI and negative for HCC based on the post-contrast sequences. However, this was not statistically evaluated as a control group, as there were other lesions that had proven HCCs on the same scans.
DISCUSSION
Six-monthly surveillance US is currently the recommended protocol for HCC screening.(12,14) US is widely available, quick, cost-effective and does not involve radiation or medications. The sensitivity of US is invariably limited by factors inherent to the operator and patients; some of these factors are not modifiable, such as patient habitus, fatty liver and macronodular cirrhosis.(5,15) MR imaging is usually reserved as an imaging modality for lesion characterisation after a focal abnormality has been detected on screening US.(16)
MR imaging is generally not used as a population-based screening modality owing to its high cost, limited availability and long procedure time. There are also potential long-term adverse effects of gadolinium-based MR imaging contrast agents, in terms of deposition in human tissues and nephrogenic sclerosing fibrosis.(9) However, it is clear that MR imaging has advantages over US in terms of lesion detection and less inter-operator variability.(17) Furthermore, in Singapore, where MR imaging is generally available, there is potential for it to be considered as a screening tool if it shows significant superiority over US.
Screening MR imaging using a hepatocyte-specific contrast agent, such as gadoxetate disodium (Primovist), has been advocated owing to its high sensitivity and specificity for the detection of HCC when compared to US.(18) However, abbreviated MR imaging protocols that rely on contrast-enhanced imaging take at least 20 minutes for optimal biliary excretion, which translates into more scan time, and there is a potential for gadolinium toxicity, as alluded to earlier. Non-contrast MR imaging is a more attractive alternative, as it generally requires a shorter scan time with fewer imaging acquisitions, and obviates the risks of contrast allergy and gadolinium toxicity.
The findings of our retrospective study are encouraging, achieving 88.9% sensitivity for HCCs seen on both T2-W FS and DWI. This sensitivity is higher than that reported for US in an earlier meta-analysis (63%),(4) and is comparable to that of non-contrast MR imaging in the study by Han et al.(7) The sensitivity of US for the detection of HCC in our study was 79.5%, which is within the reported range of the meta-analysis.(4)
In our study, non-contrast MR imaging was able to detect more than half of the HCCs (65%) that were not detected on US. Conversely, non-contrast MR imaging failed to detect only 7% of HCCs seen on US. This further suggests that a majority of HCCs missed on US could potentially be visualised on non-contrast MR imaging.
One observation from this study concerned the evaluation of cirrhotic liver on non-contrast MR imaging. It was noted that some cirrhotic livers in our study showed diffuse heterogeneous signal on T2-W FS sequences, rendering evaluation for focal T2-abnormality difficult, particularly for smaller HCCs.
Interpretation of DWI in cirrhotic liver was also challenging, given that confluent fibrosis can also show areas of restricted diffusion that may obscure focal lesions.(19) Our study suggested that the presence of imaging features of cirrhosis slightly decreased the sensitivity of non-contrast MR imaging. However, it still remains significantly higher than that of US. Liver cirrhosis is also a known confounding factor reducing the sensitivity of DWI sequences for detection of HCC, particularly the well-differentiated or early variants.
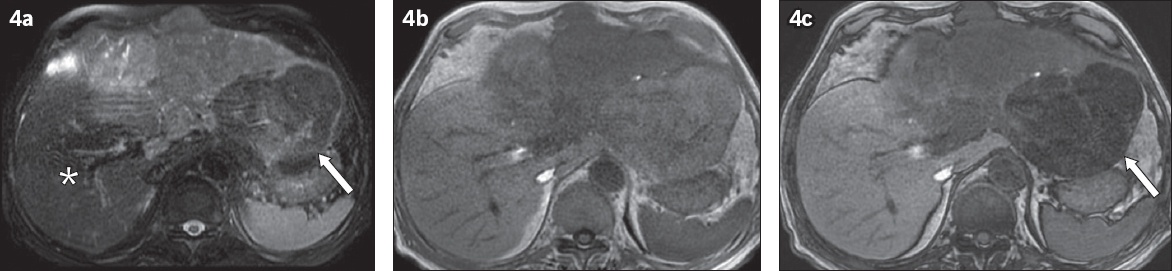
Our study also suggested that fat-containing HCCs were a potential pitfall for non-contrast MR imaging. Three patients had fat-containing HCCs that were completely undetected on T2-W FS and DWI sequences (
Fig. 4
Partial fat-containing hepatocellular carcinoma (HCC) appeared similar to normal liver parenchyma on T2-W fat-saturated (FS) sequences. (a) Axial T2-W FS MR image showed large T2-W intermediate signal mass occupying most of the left lobe and part of the right lobe. Note that a component of the tumour in the left lobe containing fat (arrow) had similar signal to the normal hepatic segment VI/VII liver parenchyma (asterisk), and could have been easily overlooked on T2-W FS sequence. (b) In-phase and (c) out-of-phase sequences showed a component of the HCC with marked hypointense signal on the out-of-phase sequence (arrow), in keeping with intratumoral fat.
While other non-contrast MR imaging sequences, such as T1-W gradient in- and out-of-phase sequences and a 3D T1-W sequence with fat saturation, may be helpful, the two sequences (T2-W FS and DWI) evaluated in our study are the two key sequences used in clinical practice and are important for evaluation of ancillary features of malignancy in accordance with the Liver Imaging Reporting and Data System (LI-RADS).(11)
Theoretically, the addition of T1-W in- and out-of-phase sequences to the non-contrast MR imaging protocol could improve the performance of non-contrast MR imaging by detecting fat-containing HCCs.(7,10) However, this was not the case in our study, as we observed that all fat-containing HCCs were visible on either both T2-W FS sequences and DWI or neither of them. This meant that T1-W in- and out-of-phase sequences would be useful only when read independently, and the addition of this sequence would have had limited impact, at least in our study, increasing the HCC detection sensitivity by only 2.8% (3/108 HCCs). Moreover, fat-containing HCCs are known to be difficult to visualise in small HCCs less than 1.5 cm in size and are visualised only in 3.5% of larger HCCs.(21) Based on a recent study of ancillary features on LI-RADS, it was found that intralesional fat depiction carried a sharply lower sensitivity (30.9%), albeit higher specificity for HCC, when compared to T2-W (62.2%) MR imaging or DWI (54.8%).(22) For use as a screening modality, sequences that carry greater sensitivity are preferred.
Our study had a few limitations. By selecting a patient cohort from the cancer registry, readers were not blinded to the diagnosis of HCC, although they were blinded to the imaging findings. We also lacked a control group without HCC, owing to which we could not perform a complete assessment of diagnostic performance that would have included specificity, negative and positive predictive values as well as accuracy. However, it should be noted that T2-hyperintensity and restricted diffusion (positive non-contrast MR imaging) is not specific for HCC and can also be seen in metastatic lesions and other primary liver malignancies such as angiosarcomas and cholangiocarcinomas.(23-25) Furthermore, in the context of screening, the intent was to utilise the diagnostic tool that conferred the highest possible sensitivity, within resource constraints.
Owing to the retrospective nature of our study, we were unable to compare both MR imaging and US performed in the same sittings, which would have been ideal. However, this was not practically feasible, as an interval time period between the two modalities could have confounded the results. Employing this method of comparison could also result in positive selection bias, as MR imaging is used to further evaluate abnormal US scans in usual clinical practice. We tried to reduce this confounding effect by ensuring that all MR imaging was performed within three months of US. The fact that our results remain comparable to those reported in the literature suggested that the confounding effect was likely to be non-significant. Furthermore, the MR images were performed on two different systems of different magnetic field strengths, which could result in differing image quality and potentially affect lesion detection.
Finally, for MR imaging to be advocated as a screening tool to replace US, proper cost-benefit ratio analysis that compares the lower cost of US against the higher sensitivity of MR imaging should be performed. The target group should also be more specifically defined. For example, MR imaging may be advocated for patients with cirrhosis secondary to chronic viral hepatitis, which is deemed to be ‘super high risk’,(26) to increase the pre-test probability and the effectiveness of a screening programme.
In conclusion, our retrospective analysis demonstrated that non-contrast MR imaging using T2-W FS and DWI sequences was significantly more sensitive than US for HCC surveillance of at-risk patients. This may serve as a basis for a larger, cohort case-control prospective study, in which a prospective, head-to-head comparison with US as a screening tool can be formally evaluated.


