Abstract
INTRODUCTION
Invasive prenatal diagnosis (IPD) has long been used to prenatally diagnose Down syndrome (DS), but it is associated with a small risk of miscarriage. Noninvasive prenatal testing (NIPT) is a highly sensitive screening test using cell-free DNA in maternal blood for detection of DS without the risk of miscarriage, but it confers a small risk of false-positive and false-negative results. The implementation of these procedures into clinical practice requires an understanding of stakeholder preferences.
METHODS
A total of 69 health professionals (HPs) and 301 women took part in a discrete choice experiment (DCE) in which preferences for four prenatal test attributes – accuracy, time of results, risk of miscarriage and amount of information provided – were assessed. Conditional logit regression was used to analyse the data. Data on demographics and ranked preferences for test attributes was collected, and a direct choice question regarding NIPT, IPD or neither test was posed to participants.
RESULTS
The women showed a preference for test safety, whereas HPs prioritised test accuracy above all other attributes. When offered a direct choice of NIPT, IPD or neither test, women aged 35 years and older, those with previous miscarriage or who knew a child with DS were more likely to choose NIPT. Chinese women preferred NIPT, whereas Indian women preferred IPD.
CONCLUSION
Our data highlights the need for patient-specific counselling, taking into account previous experiences and cultural factors. Since women and HPs prioritise different test attributes, it is essential that HPs recognise these differences in order to provide non-biased counselling.
INTRODUCTION
Combined first trimester screening (cFTS) based on serum and ultrasonography markers is offered to women to identify those who are at increased risk of carrying a baby affected with Down syndrome (DS).(1) cFTS has a detection rate of 84%–90%(2-4) and a false-positive rate of 5%. Women found to have an increased risk of aneuploidy have traditionally been offered invasive prenatal diagnosis (IPD) to confirm the result. Although IPD provides an extremely accurate diagnosis, there is a procedure-related risk of miscarriage of up to 1%.(5) Noninvasive prenatal testing (NIPT) for DS using cell-free DNA (cfDNA) from maternal blood was developed in 2008(6,7) and became commercially available three years later.(8) There are now numerous companies providing NIPT for the most common aneuploidies(9) and selected microdeletions.(10,11) NIPT for DS is a highly accurate screening test with a sensitivity of 99.2% and a specificity of 99.1%,(12) and it can be performed from ten weeks of gestation. There is still a small risk of a false-positive result, primarily due to the fact that the cell-free fetal DNA component of cfDNA is derived from the placenta(13) and confined placental mosaicism may be a source of additional chromosome 21 sequences. Consequently, it is essential to remember that NIPT is only a screening method and a subsequent invasive test is recommended to confirm a positive result.
Some countries are beginning to implement the technology into their national health programmes,(14,15) and recent research highlights differences in how NIPT is being implemented throughout the world.(16) Unlike many countries, Singapore does not have a free healthcare system; all patients have to contribute to the costs of their care, either as private or subsidised patients. cFTS is offered to all women in Singapore as part of their routine care, at a cost of approximately SGD 130 for subsidised patients and SGD 270 for private patients. NIPT was introduced to Singapore in 2013 and is becoming more widely used, although it is currently available only to private patients. The costs for NIPT tests range from SGD 1,100 to SGD 2,500, making it far more expensive than cFTS.
When implementing a new service into clinical practice, stakeholders’ opinions and preferences must be taken into consideration to ensure that the needs of both the service users and providers are met. Effective informed consent would require a combination of input from the prospective parents and health professionals (HPs), in order for effective patient counselling programmes and educational materials to be developed. In addition, it is important that cultural and social factors are taken into consideration when advising women of their options for prenatal testing.(17,18) For example, a study carried out in Europe and Asia demonstrated that women from Northern Europe believed that choices for prenatal testing should reflect parental choice. However, in Southern Europe, China and India, the majority of women felt that all women should have a prenatal test.(19) Consequently, it is important that culturally specific data is gathered to inform the ongoing implementation of NIPT in Singapore.
One method for evaluating preferences is the discrete choice experiment (DCE). DCEs are based on the premise that any service or product can be evaluated by different levels of its characteristics, described as attributes.(20) Individuals are presented with a series of scenarios, each containing two or more choices involving the attributes of interest, presented at different levels. For each scenario, they are asked to choose their preferred option. The responses are modelled statistically, and the importance of each attribute to an individual’s decision-making and willingness to make a trade-off between attributes is then assessed. DCEs are an essential aid to predict test uptake and can be used to guide policy implementation. Previous research using DCEs to examine preferences for prenatal tests, including NIPT, has shown that the main concern of women is the safety of their baby, whereas the primary consideration for HPs is the accuracy of the test.(21,22) A DCE comparing the preferences of women and HPs from nine different countries, including Singapore, has recently been published.(23) Herein, we report detailed findings of the preferences of women and HPs for NIPT from the Singapore cohort, with the aim of gaining an understanding of the challenges women and HPs may face upon widespread implementation of the test in Singapore. Preferences for four prenatal test attributes – accuracy, time of results, risk of miscarriage and amount of information provided – were assessed.
METHODS
The study design and analysis followed current guidelines for conducting DCEs.(24,25) The study was approved by the National Healthcare Group Domain Specific Review Board, Singapore (DSRB no. D/00803).
We recruited a convenience sample of pregnant women attending two maternity clinics for routine clinical care at National University Hospital (NUH), Singapore. The women were approached to participate in the study while waiting for their clinical appointment. Those who agreed to participate were given an information sheet describing DS, and the tests for DS screening and diagnosis. A researcher was available to discuss any questions regarding the questionnaire and study. Using convenience sampling, we also recruited HPs who were providing antenatal care and counselling for DS screening and diagnostic testing from NUH and Singapore General Hospital. The recruited HPs from the two centres were requested to complete Section A (i.e. DCE choice sets) and Section B of the questionnaire, as well as some demographic questions. Questionnaires were also handed out to Singaporean HPs at the Singapore International Congress of Obstetrics and Gynaecology 2013 meeting. Suitable participants were approached in person and requested to either complete a hard copy of the questionnaire or participate using a Survey Monkey questionnaire online. The sample size was estimated based on previous experience with DCE studies,(21) which suggested that a sample size of 200–300 women and 50–100 HPs was sufficient to determine the main effects and identify significant differences in preferences for test attributes between subgroups.
The questionnaire consisted of three sections: Section A – DCE choice sets; Section B – structured questions related to IPD and NIPT; and Section C – demographic questions (Appendix 1). The questionnaire was modified from that used in a previous United Kingdom (UK) DCE study(21) that has been shown to generate plausible results. The attributes used in our DCE were the same as those used in the UK study, except for the attribute levels, which were updated in the present study to match the current clinical attributes of NIPT and invasive testing. The chosen attributes covered the major differences between NIPT and invasive testing, namely accuracy (levels of 95%, 99% and 100%), time of results (10 weeks, 12 weeks and 16 weeks of gestation), risk of miscarriage (small [1%] or none) and amount of information provided (trisomies 21, 18 and 13 only, or these three aneuploidies plus additional information on other chromosomal abnormalities). As detailed in the study by Hill et al,(23) the number of possible combinations of attributes and levels was reduced statistically from 32 scenarios (23 × 22) to nine. After an internal consistency check, an extra choice set with one obviously superior test was included, resulting in a total of ten pairwise choice sets. Each attribute occurred with equal frequency across the choice sets and there was no overlap in attribute levels within each choice set. Both the women and HPs were asked to select Test A, Test B or neither for their respective choice sets.
The structured questions relating to prenatal testing included a direct choice between NIPT and an invasive test. NIPT was described as a blood test that was 99% accurate, able to detect DS, Edwards syndrome and Patau syndrome, and having no risk of miscarriage. It was also explained that following a positive result, an invasive test with a small risk of miscarriage would be advised. The invasive test was described as 100% accurate, able to test for DS, Edwards syndrome or Patau syndrome, and able to provide additional information about rare genetic conditions that may cause other health problems. It was also stated that the test was invasive and had a 1% risk of miscarriage. The women were also asked whether they had ever undergone or would undergo prenatal testing for DS, their reasons for accepting or declining prenatal testing, and whether they had had a previous miscarriage. They were asked to rank six attributes in order of ‘most important’ to ‘least important’. Demographic questions for the women included age, ethnicity, educational level and number of children, and those for HPs included job title, number of years in practice, age and gender. The questionnaire took approximately 20 minutes to complete.
A conditional logit regression model was used to analyse the DCE data for both the women and HPs.(26) Mean centred coding was used for the attributes, ‘accuracy’ and ‘time of results’, while effect coding was used for the attributes, ‘risk of miscarriage’ and ‘amount of information provided’. A constant term was included in the model to allow for the choice of neither test.(27) Coefficients generated by the regression analysis were given a ‘+’ or ‘–’ sign to indicate the direction of the preference for each attribute. The significance and size of the coefficients could be used to estimate the relative importance of the attributes. Willingness to accept a trade-off was measured using the ratio of two coefficients (marginal rates of substitution [MRS]). These MRS values allowed for the comparison of different attributes using a common scale.(24) Positive coefficients for the attributes, accuracy, amount of information provided and risk of miscarriage were anticipated, and participants were expected to prefer tests that provide higher accuracy, more information and greater safety. A negative coefficient for time of results was expected, indicating a preference for receiving earlier test results.
The preferences of the women and HPs were compared. Additional subgroup analyses for women were performed to examine differences between the women based on: type of healthcare (private vs. subsidised); age (< 35 vs. ≥ 35 years); religious faith; ethnicity; history of miscarriage (previous miscarriage vs. none); and personal experience with DS (knowing or having a child with DS vs. not knowing or having a child with DS). All analyses were carried out using Stata Statistical Software 12.0 (StataCorp LP, College Station, TX, USA). A p-value < 0.05 was considered statistically significant.
RESULTS
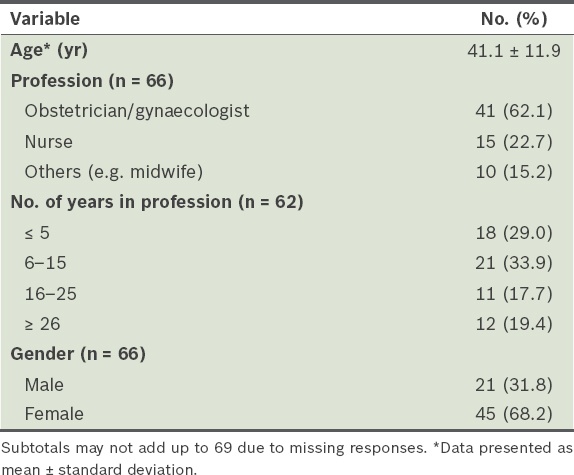
Overall, 308 women were recruited from NUH. Of these, seven women were excluded from the study; two failed to correctly answer the internal consistency question and five did not complete all the choice sets. The mean age of the 301 women included in the study was 30.7 ± 4.97 years. A total of 74 HPs were recruited, but five were excluded – one due to an incorrect consistency question and four who did not complete the choice sets. The vast majority of HPs who responded were obstetricians/gynaecologists, as most of the patient counselling for prenatal testing in Singapore is delivered by obstetricians. The demographic data of the women and HPs is summarised in Tables
Table I
Demographics of the women.
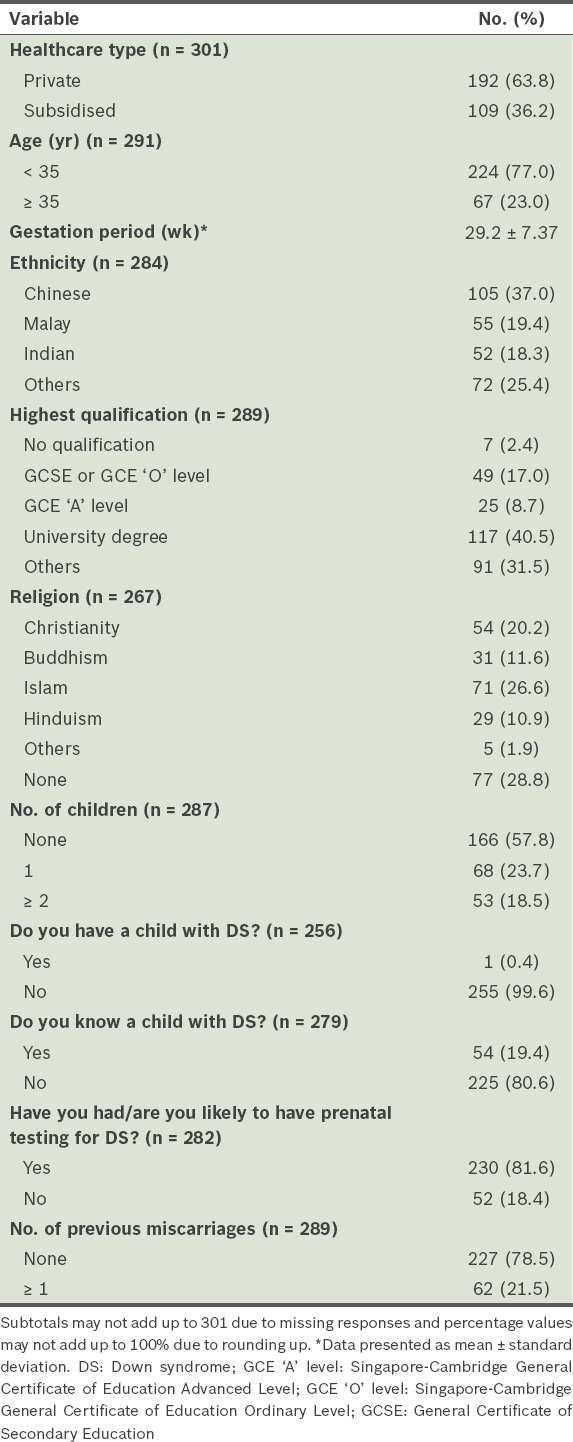
Table II
Demographics of the health professionals.
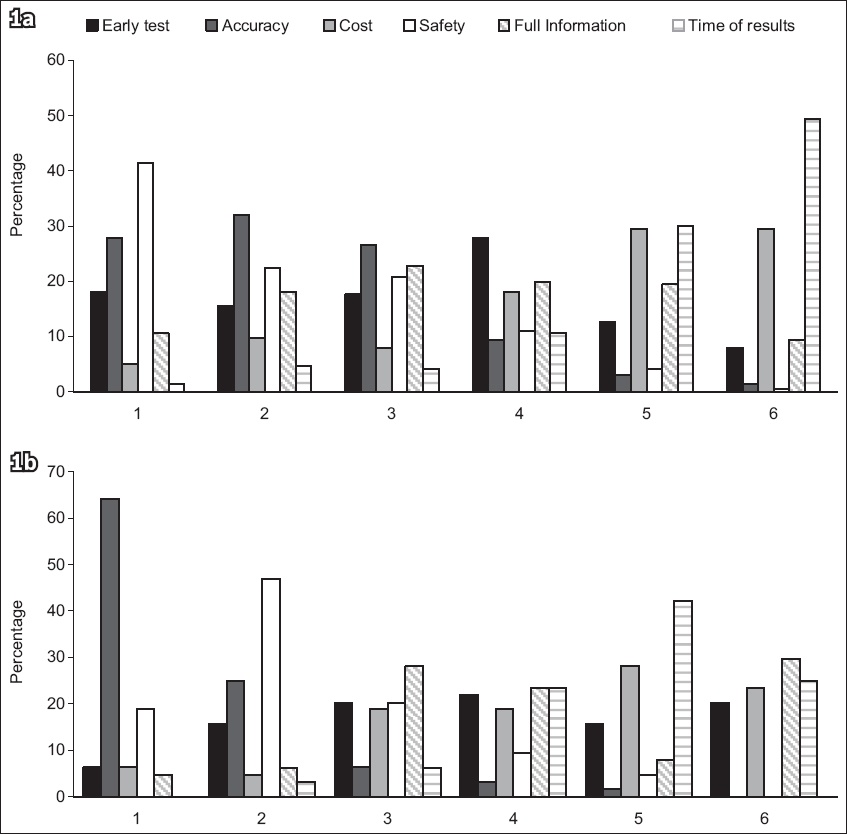
Among the 237 women who ranked the attributes, 98 (41.4%) rated safety as the most important, with accuracy as the second highest rated attribute. Almost half of the women (49.4%) ranked time of results as the least important aspect of the test, with cost being the second least important attribute (
Fig.1
Bar charts show ranked preferences for six prenatal test attributes among (a) women and (b) health professionals, with test attributes ranked in order of preference from 1 (highest priority) to 6 (lowest priority).
All four attributes examined using the DCEs (i.e. accuracy, time of results, risk of miscarriage and amount of information provided) were important to women in their decision-making process (Appendix 2,
Table III
Subgroup analysis of women’s preferences.
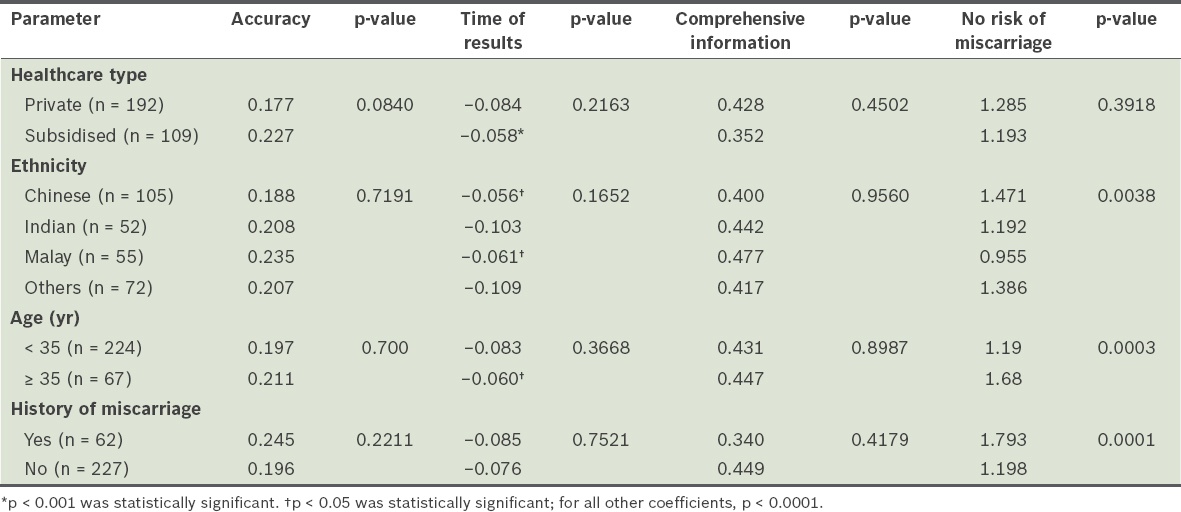
Table IV
Results of conditional logit regression analysis comparing women who would and would not choose prenatal testing.
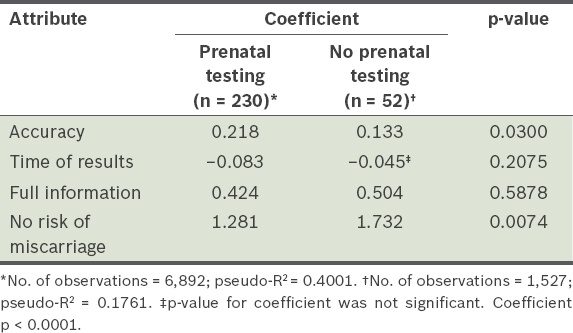
The results of the subgroup analysis are summarised in
Overall, of the 282 women who responded to the question, “Have you had/are you likely to have prenatal testing for DS?”, 230 (81.6%) said that they would choose to undergo some form of prenatal testing, while 52 (18.4%) said that they would not (
When given a direct choice among IPD, NIPT or neither test, 41.4% (n = 116) of the 280 women who responded opted for IPD, 47.1% (n = 132) preferred NIPT and 11.4% (n = 32) said that they would not take either test (
Table V
Results of conditional logit regression analysis comparing women who would choose NIPT, IPD or neither.
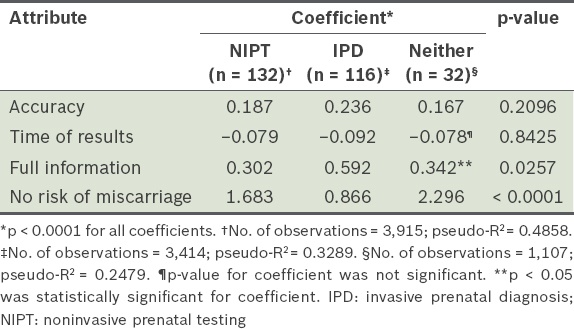
Fig. 2
Bar chart shows subgroup analysis for women giving a response to a direct choice between IPD (invasive prenatal diagnosis), NIPT (noninvasive prenatal testing) or neither test. DS: Down syndrome.
When given a direct choice, 55.1% of HPs preferred to offer NIPT, 39.3% preferred IPD and 5.8% would not recommend either test. Obstetricians favoured NIPT over IPD (60.4% vs. 33.3%, p = 0.036; data not shown), while nurses and midwives preferred IPD over NIPT (64.7% vs. 29.4%, p = 0.038).
DISCUSSION
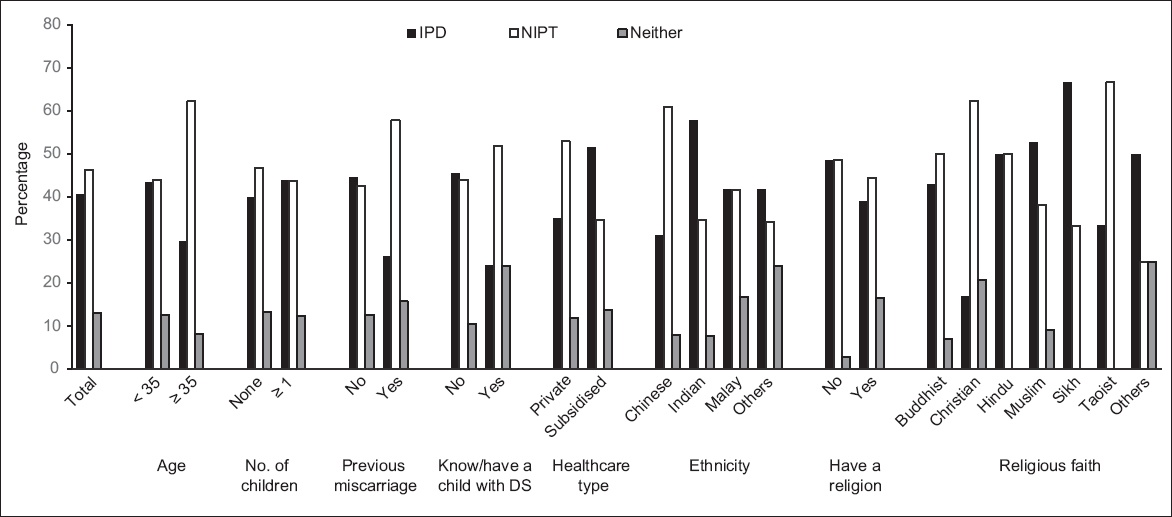
Overall, we found that when making decisions regarding prenatal testing, women in Singapore, like those in other countries,(21-23,28) place greater emphasis on test safety, while HPs place higher value on accuracy and early testing. We found that women who had a previous miscarriage, were aged ≥ 35 years or knew a child with DS gave higher priority to a test that does not carry a risk of miscarriage. Chinese women showed a greater preference for test safety compared to the other ethnic groups examined. Given a direct choice between NIPT and IPD, previous miscarriage, age ≥ 35 years, knowing a child with DS, Christian faith and Chinese ethnicity were indicators for selection of NIPT. Interestingly, although women receiving both subsidised and private healthcare have demonstrated no significant difference with regard to preferences for any of the test attributes, private patients would prefer to opt for NIPT, while subsidised patients would prefer IPD when given a direct choice.
Clear differences in preferences for test attributes between women and HPs highlight that when counselling women about NIPT, HPs should keep in mind that women’s views and preferences may differ from their own. In addition, to ensure that their women patients consider beyond test safety and to support informed decision-making, HPs should make an effort to discuss the various attributes of the prenatal test options available and highlight the limitations of these tests. HPs’ preference for accuracy over all other attributes for a new prenatal test may be indicative of a greater understanding about the limitations of NIPT, and the risk of both false-positive and false-negative results from NIPT. Good counselling is needed to ensure that autonomous informed consent is promoted.(29)
Three-quarters of the women in this study indicated that they would undergo some form of prenatal testing. Of those who responded, when given a choice between NIPT, IPD or neither test, 47.1% would choose NIPT, 41.4% would choose IPD and a sizable proportion (11.4%) would choose neither test. In comparison to many other countries,(23) both HPs and women in Singapore were more likely to choose an invasive test over NIPT when given a direct choice. The decision to opt for IPD over NIPT appears to be driven by a preference for having as much information as possible, since women who chose IPD were found to place greater value on having full information than women who chose NIPT or no test. There is evidence that women from some other countries also show a preference for IPD over NIPT,(23) or would prefer not to have any prenatal testing.(23,30,31) Therefore, while it is likely that many women will decide to have NIPT, it is important that HPs discuss alternatives to NIPT, such as invasive testing and the option of no testing at all, during pre-test counselling.
Both the women and HPs in our study emphasised comprehensive information from a prenatal test for decision-making, and it appears that many would choose IPD over NIPT to maximise the information available to them. Recent developments in sequencing technology and algorithms have allowed for analysis of a number of additional conditions, including sex chromosome abnormalities,(32,33) microdeletions(34,35) and rare aneuploidies (such as trisomies 22 and 16).(36) However, the sensitivity of these tests is unknown, and they have not been validated in clinical practice, which will inevitably increase the false-positive rate. Thus, there continues to be a need for confirmatory invasive testing.(37) Furthermore, HPs need to consider the additional counselling that will be required, both pre- and post-test, if additional findings are integrated into NIPT. At present, in Singapore, samples taken for IPD are karyotyped and this can detect major trisomies and other rearrangements that are microscopically visible. A chromosomal microarray is only performed if fetal sonographic anomalies raise suspicion of a microdeletion syndrome (e.g. cardiac anomalies in DiGeorge syndrome). Therefore, obstetricians in Singapore generally have to counsel only patients on trisomies 21, 18 and 13. In addition to the additional knowledge required for an expanded panel, there are time constraints for obstetricians and very few trained genetic counsellors in Singapore. A study in Hong Kong showed that patients were able to understand the limitations of NIPT, with more than 90% of patients appreciating the potential for false-negative and false-positive results, but being less knowledgeable on the more complicated aspects.(38) In order to provide stakeholders with the comprehensive knowledge they desire, a greater range of disorders will have to be added to the NIPT panels. There have been concerns that this would result in women having to assimilate too much information prior to giving proper informed consent. However, there is also a high chance of detecting a maternal abnormality (e.g. the mother may be a carrier of a sex chromosome abnormality(39) or a DiGeorge mutation(40)). Some cases of maternal malignancy have even been detected by NIPT.(41,42) Depending on the platform used, which affects the likelihood of these additional findings, some or all of these eventualities must be explained to the patient.
We have shown that even in a small country such as Singapore, there are significant differences in women’s views on prenatal testing, indicating that individualised, parent-directed counselling is required. For example, older mothers and those who have had a previous miscarriage are particularly concerned about safety and, thus, may be more inclined to opt for NIPT. Chinese women tended to give the highest priority to the safety of the baby, as seen in both the discrete choice analysis of test attributes and their preference for NIPT over IPD, whereas Indian women preferred IPD over NIPT. In the present study, women of Chinese ethnicity were slightly older than the rest (31.4 years vs. 30.4 years, p = 0.044), and it is thus possible that older age could have contributed to their preference for NIPT. It is also probable that cultural factors played a part in these preferences. In order to make an informed choice, it is essential that women understand the tests that they are being offered. As demonstrated by Farrell et al,(17) Latina women in the United States with poorer English competency (and lower levels of education, in general) had a lower uptake of NIPT and also lacked understanding that NIPT does not test for all chromosomal abnormalities. Since Singapore is ethnically diverse, it would be important to consider the provision of patient information leaflets in multiple languages. In addition, counselling should be provided by a multilingual HP, and if that is not the case, a translator should be present.
Although the women in the present study stated that the cost of the test was not a high priority, when faced with the reality of an expensive test, it is possible that there will be a limit to the price that they are willing to pay. NIPT is an expensive test, costing SGD 1,100–2,500. In Singapore, an invasive procedure costs approximately SGD 1,100 for private patients; therefore, there is not much difference in cost between NIPT and the slightly less expensive IPD. However, the cost of an invasive test can be covered using funds from the compulsory Medisave savings scheme that all Singaporeans must contribute to, whereas NIPT is an entirely out-of-pocket expense. As discussed earlier, although there was no difference in the preferences of subsidised and private patients for DS test attributes, when given the choice of an IPD or NIPT, the majority of subsidised patients would choose IPD while the majority of private patients would choose NIPT. There are many possible explanations for this, one of which is the perceived higher cost of NIPT, since it is a new technology. A study in Hong Kong showed that significantly more women would opt for self-funded NIPT as a contingent second-line test, rather than a first-line test, after a cFTS (freely available in public hospitals).(38) In Singapore, a cFTS costs SGD 270 for a private patient and SGD 130 for a subsidised patient. Thus, it is possible that the same outcome would be seen in Singapore if patients were given these options.
In our clinic, a contingent screening model is being implemented, whereby women with ‘low’ cFTS risk (> 1:1,000) are advised that no further screening is necessary. Those with intermediate or high risk (range 1:2–1:1,000) are counselled on the options of NIPT or IPD. Studies have shown that the introduction of NIPT decreased the likelihood of a patient declining further testing following a high-risk cFTS screen. A study by Chetty et al reported a decrease from 52.8% to 21.2%,(43) while other studies have shown a significant reduction in invasive testing subsequent to the availability of NIPT.(43-45) In light of our finding that there is an approximately equal preference for NIPT and IPD, it will be interesting to see how the widespread introduction of NIPT in Singapore will affect women’s interest in IPD. Additionally, it will be interesting to see whether women’s uptake of cFTS will change with more widespread use of NIPT.
There were several limitations to our study. Firstly, the study cohort was a convenience sample from the population who participated voluntarily. This may have resulted in bias toward those with strong views on prenatal testing and the results may thus not be generalisable to the entire population. For example, 40.5% of women who took part were highly educated and hence, their views may not be representative of the Singapore population. Previous studies have also shown that interest in and uptake of NIPT is greater among women with higher levels of education.(17,46) Secondly, only 37.0% of women who participated were Chinese, and this does not match the overall Singapore population, which is 74.3% Chinese. In addition, a large proportion of the Chinese women who visited our clinic were originally from mainland China and did not have English as their first language; since the questionnaire was set in English, participation would have been more challenging for these women. Thirdly, participants were recruited from only two centres, and thus, their preferences may not be reflective of those of women in the whole country. Considerable variation in the uptake of DS screening has been demonstrated among different regions in the UK(47) and Netherlands;(48) although this is less likely to be an issue in Singapore, given its small size and the small number of maternity units (only nine) in the country, the demographics of the women attending these centres may nevertheless vary. Fourthly, although the HPs included in the study completed all the DCE choices, many did not answer all of the demographic data questions, and we were thus unable to conduct a detailed subgroup analysis for this group of participants. Finally, only four attributes of prenatal tests were evaluated. In reality, decisions about healthcare often involve multiple factors, with cost likely to play a key part in decision-making. Also, when faced with a real-life situation rather than a hypothetical one, women may opt for a different course. We did not, however, explore any of the reasons behind the participants’ preferences or investigate how they viewed the tests, although these would be interesting areas for future studies.
In conclusion, we have found that, in general, women in Singapore who opt to undergo DS screening have a preference for safety, while HPs prioritise accuracy of results over other aspects. It is essential that HPs are aware of the differences in women’s preferences in order to provide non-biased counselling. In addition, HPs need to give patients adequate information on the full range of tests available, as well as spend time explaining the benefits and limitations of each option while showing sensitivity to the cultural differences among their patients.
SMJ-58-298-appendices.pdf


