Abstract
INTRODUCTION
Recent studies reported that laparoscopic pancreatoduodenectomy (LPD) is associated with superior perioperative outcomes compared to the open approach. However, concerns have been raised about the safety of LPD, especially during the learning phase. Robotic pancreatoduodenectomy (RPD) has been reported to be associated with a shorter learning curve compared to LPD. We herein present our initial experience with RPD.
METHODS
A retrospective review of a single-institution prospective robotic hepatopancreaticobiliary (HPB) surgery database of 70 patients identified seven consecutive RPDs performed by a single surgeon in 2016–2017. These were matched at a 1:2 ratio with 14 open pancreatoduodenectomies (OPDs) selected from 77 consecutive pancreatoduodenectomies performed by the same surgeon between 2011 and 2017.
RESULTS
Seven patients underwent RPD, of which five were hybrid procedures with open reconstruction. There were no open conversions. Median operative time was 710.0 (range 560.0–930.0) minutes. Two major morbidities (> Grade 2) occurred: one gastrojejunostomy bleed requiring endoscopic haemostasis and one delayed gastric emptying requiring feeding tube placement. There were no pancreatic fistulas, reoperations or 90-day/in-hospital mortalities in the RPD group. Comparison between RPD and OPD demonstrated that RPD was associated with a significantly longer operative time. Compared to open surgery, there was no significant difference in estimated blood loss, blood transfusion, postoperative stay, pancreatic fistula rates, morbidity and mortality rates, R0 resection rates, and lymph node harvest rates.
CONCLUSION
Our initial experience demonstrates that RPD is feasible and safe in selected patients. It can be safely adopted without any compromise in patient outcomes compared to the open approach.
INTRODUCTION
Tumours involving the periampullary region are often treated with pancreatoduodenectomy (PD), also known as the Whipple procedure. This has traditionally been performed via laparotomy due to the complexity of the procedure. In recent years, increasing reports of laparoscopic pancreatoduodenectomy (LPD) have been reported from highly specialised tertiary centres.(1) Although the first report of LPD was published over two decades ago,(2) its adoption by pancreatic surgeons has been understandably slow due to the technical complexity of the procedure. Only in the past decade did its adoption began to gain traction among selected surgeons, especially in high-volume specialised centres.(3)
Recent studies from these expert centres that overcame the steep learning curve have reported encouraging results, such as decreased blood loss and shorter postoperative stay(4-6) compared to the open approach. However, concerns about the long and steep learning curve associated with LPD remain, preventing its widespread adoption. Hence, unlike distal pancreatectomies, LPD is routinely performed by relatively few surgeons worldwide. Even reports from high-volume institutions have demonstrated high conversion rates(7) and increased morbidity(8,9) compared to the open approach, especially during the initial learning phase. Hence, to bridge the high level of expertise and training required to perform the pancreato-enteric anastomosis laparoscopically, many surgeons have proposed the hybrid or laparoscopic-assisted approach as a safer alternative during the initial learning phase, whereby dissection and resection are performed laparoscopically, and reconstruction is done via a mini-laparotomy midline incision.(10,11)
The emergence of the use of robotic surgery for pancreatectomies in recent years has provided a viable alternative to the open and laparoscopic methods.(12) Robotic pancreatoduodenectomy (RPD) has been shown to afford the benefits of laparoscopy, such as decreased blood loss and shorter postoperative stay,(3) while also allowing for greater stability and precision in instrument handling.(13)
Locally in Singapore, the adoption of minimally invasive surgery for pancreatectomy has rapidly increased over the past decade. However, to our knowledge, only studies reporting on distal pancreatectomies have been published thus far. The first series of laparoscopic distal pancreatectomy (LDP) was reported from Tan Tock Seng Hospital in a small series of three patients.(14) Several larger series of LDPs have since been reported from other institutions.(15-17) Regarding robotic pancreatic surgery in Singapore, the first series was only reported recently in 2016, and subsequent series have been limited to distal pancreatectomies.(18,19)
In this study, we report our initial experience with RPD. To the best of our knowledge, this is the first study to date reporting on RPD in Singapore. In order to determine the safety of adopting RPD, we compared the outcomes with a matched cohort of open pancreatoduodenectomy (OPD) procedures performed by the same surgeon.
METHODS
We conducted a retrospective review of our single-institution prospective robotic hepatopancreatobiliary (HPB) database from 2013 to 2017. There were 70 cases of robotic HPB surgeries, of which seven consecutive RPDs were performed between 2016 and 2017. All seven cases were performed by a single surgeon (Goh BK) who had prior experience performing over 100 OPDs, 100 laparoscopic major HPB surgeries and 25 robotic HPB procedures prior to embarking on the first RPD. Suitability for RPD was determined by the surgeon, who discussed with the patient the various options that were available, including OPD, LPD and RPD. Informed consent was obtained from the patient after emphasising the advantages and limitations, including costs, of the different approaches.
In order to determine the safety of the adoption of RPD, we performed a matched 1:2 comparison between RPD and OPD by the same surgeon. Reviewing our prospective database of PDs performed between 2011 and 2017, we identified 77 consecutive PDs performed by the same surgeon. Of these, 14 patients who underwent OPD were matched in a 1:2 fashion with the seven RPDs based on characteristics such as (a) extended or standard PD; (b) type of anastomosis (pancreatogastrostomy [PG] vs. pancreatojejunostomy); (c) total pancreatectomy; (d) pancreatic consistency (i.e. soft, moderate or hard); (e) pancreatic duct size (< 6 mm or ≥ 6 mm); (d) tumour pathology (pancreatic ductal adenocarcinoma, periampullary tumour or others); and (e) patient age (> 70 years or ≤ 70 years), American Society of Anesthesiologists score and gender.
Clinicopathological data including patient demographics and relevant preoperative, intraoperative and postoperative information was obtained retrospectively from patient records. Clinical data was collected from a prospective computerised clinical database (Sunrise Clinical Manager version 5.8; Eclipsys Corporation, Atlanta, GA, USA) and patient clinical charts, while operative data was obtained from another prospective computerised database (OTM 10; IBM Corp, Armonk, NY, USA). The study was approved by the SingHealth Centralised Institution Review Board.
Resection type was classified into either standard or extended pancreatectomy based on the 2014 International Study Group of Pancreatic Surgery consensus definition.(20) Postoperative complications were graded according to the Clavien-Dindo grading system(21) and recorded regardless of length of postoperative stay or, if the patient was discharged and re-admitted, within a 30-day duration. Major morbidity was defined as any morbidity > Grade 2. Postoperative pancreatic fistula (POPF) was defined and graded according to the updated International Study Group of Pancreatic Fistula system.(22) This was defined as any amount of drain fluid with an amylase content of more than three times the upper normal limit of serum amylase or > 300 IU/L on or after the third postoperative day (POD). Patients with Grade B pancreatic fistula included those who had surgical drains kept in place for more than three weeks or who required endoscopic or percutaneous placement of new drains. Grade C pancreatic fistulas were fistulas that required reoperation, resulted in organ failure or resulted in mortality. Asymptomatic Grade A pancreatic fistulae were reported but not considered a morbidity and classified as a biochemical leak. Open conversion in this study was defined as requiring an open incision to complete the resection phase. Elective open conversion for reconstruction via a mini-laparotomy incision was termed a hybrid procedure and was not considered an open conversion.
90-day mortality was defined as any death within 90 days from surgery. In-hospital mortality was defined as any death during the index hospital stay regardless of time from surgery. 30-day re-admission was defined as any hospital admission occurring within 30 days of discharge for any condition related to the primary reason for surgery and the surgery itself.
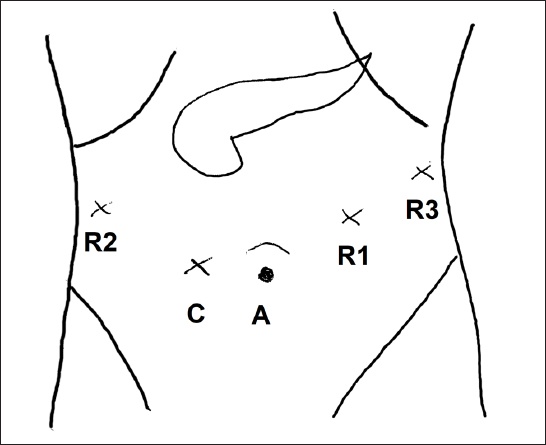
The RPD surgical technique was not standardised and evolved over time, as this represented our early experience. At the time of writing, the technique involves the placement of a subumbilical assistant port, a 12-mm right paraumbilical port for the robotic camera, an 8-mm right hypochondrium port and two 8-mm left hypochondrium ports (
Fig. 1
Diagram shows the positions of the subumbilical assistant port (A), 12-mm right paraumbilical port for the robotic camera (C), 8-mm right hypochondrium port (R2) and two 8-mm left hypochondrium ports for robotic pancreatoduodenectomy (R1 & R3).
After the completion of the resection phase, for the hybrid procedure, open reconstruction is performed via a 7–8 cm mini-laparotomy. A retromesenteric, iso-loop reconstruction is then performed. The jejunal loop is pulled through the native duodenal tunnel, and an iso-loop reconstruction is performed through an end-to-side duct-to-mucosa modified Blumgart-style pancreaticojejunostomy with internal stent, end-to-side hepaticojejunostomy and end-to-side gastrojejunostomy. For cases with PG anastomosis, a dunking PG was performed into the posterior stomach and the PG anastomosis fashioned via an anterior gastrostomy. The reconstruction technique was similar for both OPD and RPD. For the three total RPDs, the specimen was extracted via a 4–5 cm incision in the midline.
Owing to the complexity of reconstruction in PD and the fact that this was our initial experience, five out of seven RPDs in this series were performed via the hybrid approach, whereby dissection and resection were completed via the robotic approach, but an open reconstruction was performed via an 7–8 cm upper midline mini-laparotomy incision. The Da Vinci Surgical System Si (Intuitive Inc, Sunnyvale, CA, USA) was used in all cases.
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp, Armonk, NY, USA). Univariate analyses were performed using the paired t-test or Wilcoxon signed-rank test as appropriate. All statistical tests were two-sided and p < 0.05 was considered statistically significant.
RESULTS
A total of seven patients underwent RPD. Five patients underwent hybrid RPD with open reconstruction: five were standard pancreatectomies and two were extended pancreatectomies. One of the extended resections involved a wedge resection of the superior mesenteric vein and the other involved a wedge resection of the portal vein. The details of these seven patients are summarised in
Table I
Summary of the clinicopathological features and perioperative outcomes of patients who underwent robotic pancreatoduodenectomy (n = 7).
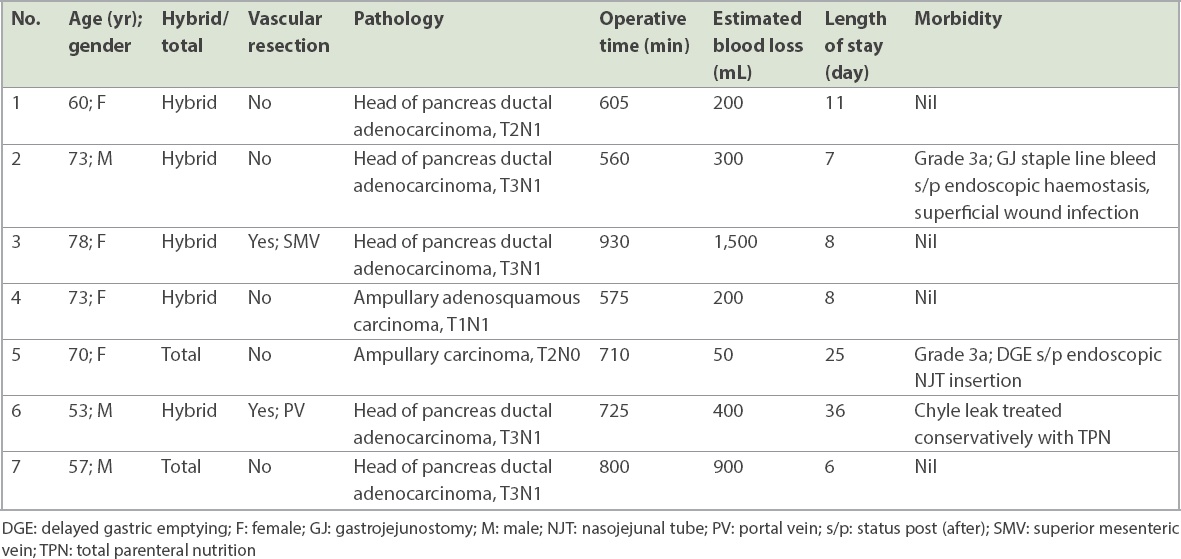
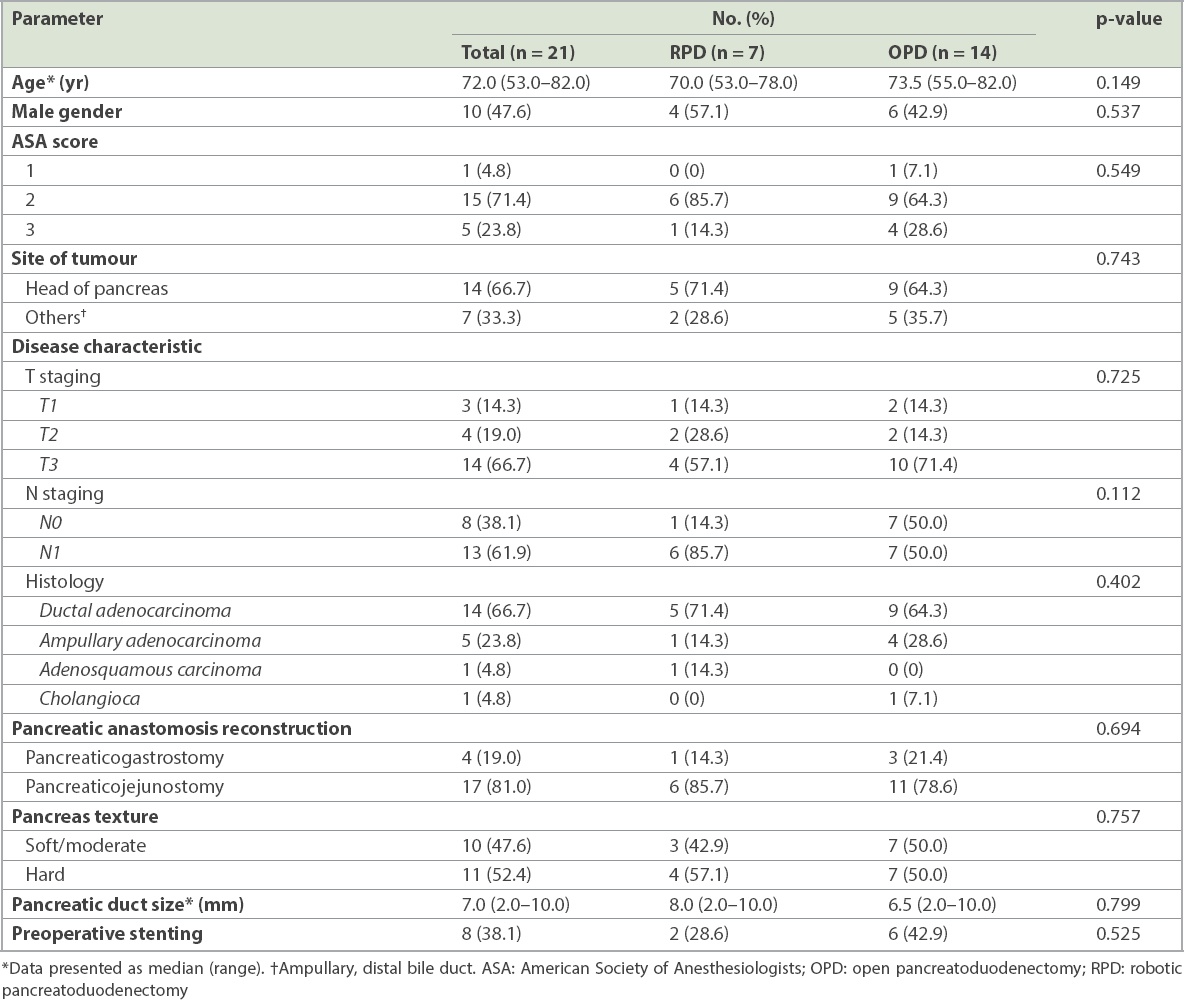
Table II
Baseline characteristics of the study patients who underwent RPD or OPD.
The clinical outcomes of the patients are summarised in
Table III
Comparison of patient outcomes between RPD and OPD.
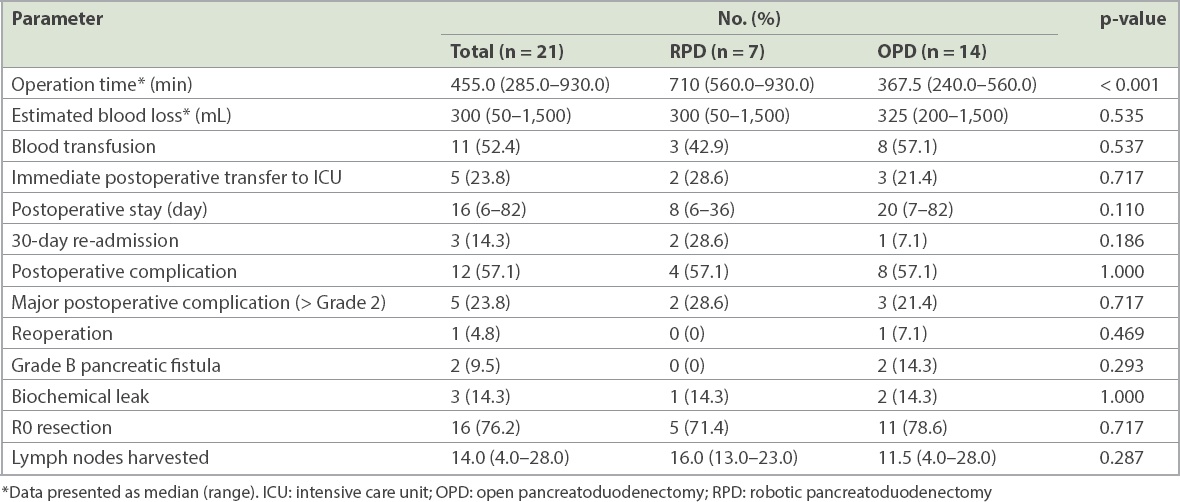
A total of five patients suffered major morbidity (> Grade 2). Of the two in the RPD group, one suffered from bleeding from the gastrojejunostomy staple line on POD 1, which was successfully treated using endoscopic haemostasis with clips. The other patient who underwent totally RPD had delayed gastric emptying that required the placement of a nasojejunal tube for feeding.
Three patients suffered major morbidity in the OPD group. All the three patients required nasojejunal tube insertion for delayed gastric emptying. One of them also developed intra-abdominal bleeding from a jejunal mesenteric vessel on POD 2 that required exploratory laparotomy and haemostasis. He also subsequently developed a Grade B pancreatic fistula requiring percutaneous drainage. Another developed pneumonia with Type 2 respiratory failure and fluid overload requiring intubation and ICU management.
None of the seven patients in the RPD group developed a POPF and two patients in the OPD group developed a Grade B POPF. One of the patients with Grade B fistulae was the aforementioned patient; the other OPD patient developed an intra-abdominal collection that required prolonged total parenteral nutrition and intravenous antibiotics. One patient in the RPD group and two in the OPD group had clinically insignificant biochemical leaks. There were no 90-day or in-hospital mortalities in the 21 patients.
DISCUSSION
Minimally invasive surgery (MIS) for PD has seen a recrudescence over the recent decade, with surgeons from many large centres attempting this highly complex procedure laparoscopically and receiving promising results.(3) Increasingly, more investigators have demonstrated LPD to be safe and even superior in terms of decreased blood loss and shorter postoperative stay(4,5) while still having similar postoperative morbidity and oncologic outcomes compared to their open counterpart.(3,4,23) The results of the first randomised controlled trial comparing OPD and LPD was recently published and demonstrated promising results.(6) Studying 32 LPD and 32 OPD procedures, Palanivelu et al found increased operative time but decreased postoperative stay and blood loss in the LPD group.(6) No difference was found in the overall complication and mortality rate, number of nodes retrieved or R0 resection rate.(6)
Nevertheless, LPD remains one of the most complex surgeries in the abdominal cavity and is technically demanding with a steep learning curve.(10,24) In addition to the complex resection, the main difficulty lies primarily with reconstruction of the pancreatic anastomosis, also known as the ‘Achilles heel’ of PD.(25) Real concerns have been raised about the potential increase in morbidity and even mortality, especially during the early learning phase, with several authors having reported a high major morbidity rate during their early learning experience.(8,26) To overcome the large gap between the open and totally laparoscopic approaches, many surgeons have adopted the hybrid technique, which has been shown to be a safe ‘in-between’ approach for surgeons first embarking on LPD.(10,11,27)
The introduction of robotic technology in the early 2000s was one of the greatest advancements for MIS over the past 20 years. Since then, robotic surgery has gained widespread utilisation in a myriad of surgical procedures ranging from urological(28) to bariatric(29) and thoracic operations.(30) More recently, MIS has been increasingly utilised in the field of hepatobiliary surgery, including pancreatic surgery.(12) Compared to conventional laparoscopy, the robotic platform provides the advantages of improved three-dimensional (3D) visualisation, greater instrument range of motion and dexterity, elimination of tremors, and enhanced ergonomics,(13) thus allowing for better fine motor movement and control.(31,32) This is especially useful for highly complex procedures such as PD. It has been reported that robotic surgery enables surgeons to perform PD with a shorter learning curve and decreased conversion rate,(3,4,23) thus serving as a valuable option for surgeons preparing to perform MIS PD. A recent multicentre study analysing robotic reconstruction during the early learning curve demonstrated that RPD did not result in increased anastomotic morbidity compared to the established open technique.(33) The results of this study suggest that unlike LPD, which has been shown in some studies to result in increased morbidity compared to the open approach(8) especially during the learning phase, RPD can be adopted safely with no increase in morbidity even during the learning phase. Another recent multi-institution study from Europe reported that RPD was associated with a significantly lower conversion rate (5% vs. 26%, p < 0.001) compared to LPD,(34) suggesting that the technical advantages of RPD over LPD, including its increased dexterity, could be useful for PD.
Several recent studies compared RPD and OPD with varying results. In general, they concur that the robotic approach results in reduced blood loss,(35-38) shorter hospital stay,(35,36,38) similar(35,36,39) or even decreased complication rates,(1,37,38) but similar mortality rates.(1,35,36,38,39) In one of the earliest studies comparing RPD and OPD, Buchs et al examined 44 RPD patients and 39 OPD patients and found significantly shorter operating time (444 minutes vs. 559 minutes, p = 0.0001) and reduced blood loss (387 mL vs. 827 mL, p = 0.0001) in the robotic group compared to the open group. No difference was observed in the complication rate, mortality rate or length of stay. A significantly higher number of lymph nodes were harvested (16.8 vs. 11.0, p = 0.02) in the robotic group, although no difference was demonstrated in the R0 resection rate.(35)
Other authors have also pooled data from these disparate studies for analysis. In the largest systematic review and network meta-analysis of OPD versus MIS PD to date involving 2,759 patients in 20 studies, Ricci et al found the robotic approach to be superior to both the open or laparoscopic approach in terms of delayed gastric emptying, postoperative stay, harvested lymph nodes and postoperative morbidity. No difference in postoperative mortality was demonstrated on pairwise comparison.(1) Peng et al, in his systematic review and meta-analysis of nine non-randomised observational studies comparing a total of 245 RPDs and 435 OPDs, found a significantly lower complication rate, margin positivity, wound infection and hospital stay in the RPD group but no difference in lymph node harvest, reoperation, operation time, delayed gastric emptying, POPF or mortality.(40) The results from this meta-analysis demonstrated that RPD performed better in terms of the negative margin rate, wound infection rate and length of hospital stay. In addition, the overall complication rate was significantly lower in the RPD group.(40) In another systematic review of 13 studies comprising 738 patients, Kornaropoulos et al found no difference in morbidity or mortality between RPD and OPD. Operative time was longer, but estimated blood loss was lower in the RPD group.(41)
Although the aforementioned studies investigated the short-term outcomes and safety of RPD, none provided direct data on long-term oncological outcomes, such as survival and recurrence.(40) This parameter has, however, been investigated in terms of lymph node harvest yield and R0 resection rates, which were separately shown to be a barometer of survival in pancreatic cancer.(42,43) Overall, although many studies demonstrated no difference in these outcomes,(36,37,44) a few showed increased nodal yield(35) and higher R0 resection rates(39,40) in favour of RPD. It has been postulated that these findings are due to the improved 3D visualisation afforded by the robotic platform, but are likely also due to the fact that surgeons who perform RPD would already have considerable experience in OPD and hence would be likely to perform more complete dissections of the mesopancreas.
Several studies have also compared the outcomes of RPD with those of LPD. Liu et al(45) studied 27 RPD and 25 LPD and found that the robotic group exhibited significantly less blood loss, reduced operative times and shorter hospital stay than the laparoscopic group. Similarly, Ricci et al concluded in their systematic review and network meta-analysis that the safest MIS PD techniques to improve outcomes involve the usage of a robot.(1) This data is unsurprising, as other studies have estimated that the learning curve for RPD is shorter than that for LPD, primarily due to the ease of learning the anastomotic skills required in PD with the robotic platform.(33,46,47) These results are important; as a recent study published by Kutlu et al demonstrated, the advantages of LPD such as shorter hospital stay and lower readmission rate only become apparent in high-volume institutions (> 25 cases per year).(48) Not unexpectedly, lower PD case volumes correlated with poorer outcomes regardless of surgical approach. It was of concern that this detrimental effect was exacerbated by the laparoscopic approach.(48)
In this study, we demonstrated that RPD can be adopted safely with a similar safety profile and short-term outcome as open PD. During this early experience, we adopted a stepwise approach to ensure patient safety, whereby we performed hybrid RPD in five patients and total RPD in two patients. Despite the lack of difference in the outcomes shown as compared to that of other studies, in large part due to the small sample size, it is possible that with increased experience and further training, better outcomes can be achieved in the future.(49,50) In our opinion, the technical advantages of the robotic platform can potentially enable more surgeons, especially those in small countries such as Singapore, to perform MIS PD and hence allow more patients to enjoy the benefits of the minimally invasive approach. Routine performance of conventional LPD, with its numerous technical limitations, would likely only be limited to a few selected surgeons practising in high-volume institutions worldwide.
The cost-effectiveness of robotic surgery and MIS in general has always been a major concern in many countries. Generally, with the increased use of expensive and frequently disposable equipment associated with these new technologies, coupled with the high investment of expensive capital equipment, the widespread propagation of MIS and robotic surgery in particular has been limited, especially in developing countries. Although general MIS, including robotic surgery, has frequently been associated with shorter hospital stays and even a decrease in morbidity(5,6) compared to the open approach, these may not be sufficient to defray the increase in intraoperative costs.(51) Moreover, it is important to note that the benefits of MIS tend to decrease and be less obvious as the complexity of the surgical procedure increases. However, most comparative studies only analyse short-term perioperative outcomes and do not include analyses of other important longer-term outcomes such as time to return to normal activity/work, long-term wound pain or discomfort, wound paraesthesia, incisional hernias, cosmesis and other quality of life measures that would be negatively affected by a long open laparotomy incision. Hence, it is not surprising that national healthcare authorities today struggle to determine the cost-effectiveness of robotic surgery for many procedures and to determine the ‘price’ at which these procedures would be worth doing.
Our study is not without limitations. Firstly, as the surgery was highly specialised and relatively new to our institution, the case volume was modest. Apart from the propensity to give rise to bias, this also limited our ability to reach any significant conclusions due to lack of power, even though the data suggested a possible underlying difference (Type II error). As such, a matched pair design was decided upon to mitigate some of these effects. In addition, although cases were matched as closely as possible, they could not be perfectly matched and hence confounding effects could be present. Nevertheless, our series presents an important first experience of RPD compared to open PD by a single surgeon and provides a roadmap for further studies to examine the safety and outcomes in a larger population.
In conclusion, our initial experience demonstrated that RPD is feasible and safe in selected patients. It can be safely adopted by surgeons who are experienced in both OPD and advanced laparoscopic surgery without compromising patient outcomes, compared to the open approach. Further studies in larger patient cohorts are needed to determine if RPD results in superior patient outcomes compared to LPD and OPD.


