Abstract
INTRODUCTION
Large, recent population-based data for evaluating the predictors of oesophageal variceal bleeding (OVB) among cirrhotic patients is still lacking. This study aimed to determine the cumulative incidence of OVB among cirrhotic patients and identify the predictors of OVB occurrence.
METHODS
Patient information on 38,172 cirrhotic patients without a history of OVB, who were discharged between 1 January 2007 and 31 December 2007, was obtained from the Taiwan National Health Insurance Database for this study. All patients were followed up for three years. Death was the competing risk when calculating the cumulative incidences and hazard ratios (HRs) of OVB.
RESULTS
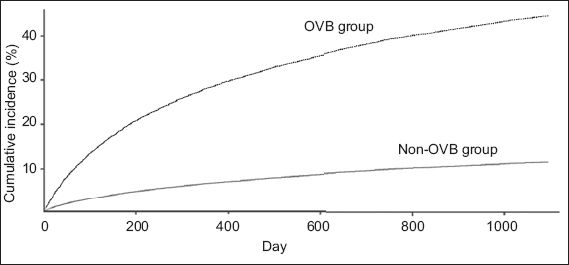
OVB was present in 2,609 patients (OVB group) and absent in 35,563 patients (non-OVB group) at hospitalisation. During the three-year follow-up period, the cumulative incidence of OVB was 44.5% and 11.3% in the OVB and non-OVB group, respectively (p < 0.001). Modified Cox regression analysis showed that the HR of OVB history was 4.42 for OVB occurrence (95% confidence interval [CI] 4.13–4.74). Other predictors for OVB occurrence included hepatocellular carcinoma (HR 1.16, 95% CI 1.09–1.24), young age (HR 0.98, 95% CI 0.98–0.98), ascites (HR 1.46, 95% CI 1.37–1.56), alcohol-related disorders (HR 1.20, 95% CI 1.12–1.28), peptic ulcer bleeding (HR 1.26, 95% CI 1.13–1.41) and diabetes mellitus (HR 1.14, 95% CI 1.06–1.23).
CONCLUSION
Cirrhotic patients have a fourfold increased risk of future OVB following the first incidence of OVB.
INTRODUCTION
Oesophageal variceal bleeding (OVB) is a frequent and potentially fatal complication in cirrhotic patients.(1) Previous studies have estimated that the crude annual incidence of the first OVB ranges from 5%–10% in non-selected cirrhotic patients without a history of previous OVB.(2-4) However, these studies included cirrhotic patients who were hospitalised two decades ago.(2-4) There is a need for recent population-based studies to determine the exact cumulative incidence of OVB among cirrhotic patients. The aim of the present study was to determine the cumulative incidence of OVB among cirrhotic patients (using competing risk analysis) and identify the predictors of the occurrence of OVB. In order to enrol a large population of cirrhotic patients, we used the National Health Insurance Research Database (NHIRD) of Taiwan.
METHODS
This study was initiated after attaining approval from the Institutional Review Board of Dalin Tzu Chi Hospital, Taiwan. As all identifying personal information was stripped from the secondary files before analysis, written informed consent was not required.
The secondary de-identified database used in the present study was from the NHIRD, which was established and maintained by the Taiwan National Health Insurance Bureau and the National Health Research Institutes. This database includes all diagnostic coding information of hospitalised patients in Taiwan and has been referenced in many articles.(5,6) Taiwan’s National Health Insurance programme was developed in 1995 and includes all citizens living in Taiwan; this programme covers more than 98% of Taiwan’s population, but their privacy is protected. Research projects requiring the use of data from the NHIRD would first need to be evaluated by the National Health Research Institutes.
This retrospective study included patients who were discharged with a diagnosis of cirrhosis (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] code 571.5 or 571.2) between 1 January 2007 and 31 December 2007. Patients who had a history of OVB in the past ten years were excluded. A total of 38,172 cirrhotic patients who did not have a history of OVB in the past ten years were enrolled. Complications of cirrhosis in the present study included hepatic encephalopathy (HE; ICD-9-CM code 572.2),(7) OVB (ICD-9-CM code 456.0, 456.20) or endoscopic procedures for OVB control (ICD-9 v3 procedure code 42.33),(8) and ascites (ICD-9-CM code 789.5, or procedure code 54.91). Comorbid medical disorders included alcohol-related disorders (ICD-9-CM codes 291, 303, 305.00–305.03, 571.0–571.3), hepatocellular carcinoma (HCC; ICD-9-CM code 155.0), peptic ulcer bleeding (PUB; ICD-9-CM codes 531.0, 531.2, 531.4, 531.6, 532.0, 532.2, 532.4, 532.6, 533.0, 533.2, 533.4 and 533.6),(9) diabetes mellitus (ICD-9-CM code 250) and end-stage renal disease (ESRD) requiring dialysis. Patients were considered to have ESRD if they were receiving long-term haemodialysis or peritoneal dialysis before admission. In Taiwan, as patients with long-term dialysis have certification to reduce the medical payment for haemodialysis, an isolated dataset from the NHIRD was used to identify patients with ESRD. Each patient was individually followed up for three years from their first hospitalisation in 2007 to the identification of OVB.
The occurrence of death may lead to informative censoring in the estimation of OVB incidence. Therefore, deaths that occurred prior to the incidence of OVB were considered as a competing risk event in our data analysis. R: A Language and Environment for Statistical Computing with the cmprsk package (R Foundation for Statistical Computing, Vienna, Austria) was used to calculate the cumulative incidence and hazard ratio (HR) in the competing risk analysis.(10-12) At a 95% confidence interval (CI), p < 0.05 indicated statistical significance. Student’s t-test was used to compare continuous variables and chi-square test was used to compare categorical variables.
RESULTS
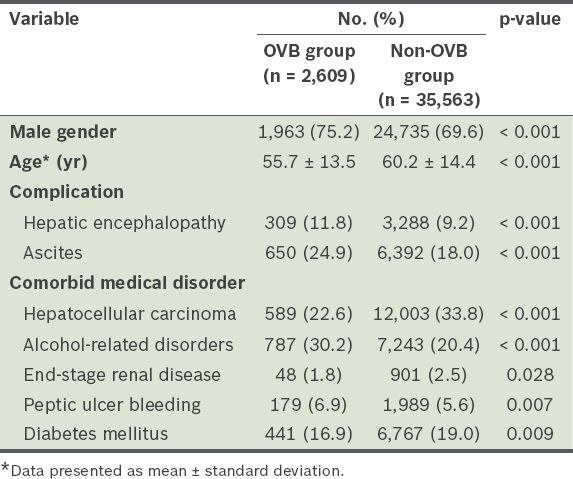
The mean age of the 38,172 cirrhotic patients without a history of OVB over the past ten years was 59.9 ± 14.4 years. Among these patients, 26,698 (69.9%) were men. At hospitalisation, 2,609 (6.8%) patients had OVB (i.e. OVB group), while 35,563 (93.2%) patients did not (i.e. non-OVB group). The six-week mortality of the OVB group was 17.1% (446/2,609). Demographic characteristics of the cirrhotic patients are listed in
Table I
Demographic characteristics of the cirrhotic patients with and without oesophageal variceal bleeding (OVB) at hospitalisation (n = 38,172).
The results of the modified Cox regression (using competing risk analysis of HR) for the occurrence of OVB are shown in
Table II
Adjusted hazard ratios (HRs) for the occurrence of oesophageal variceal bleeding (OVB) among cirrhotic patients (n = 38,172) during the three-year follow-up period.
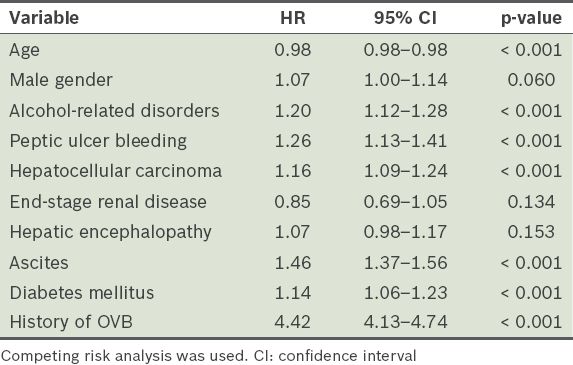
Fig. 1
Line graph shows the cumulative incidence curves of oesophageal variceal bleeding (OVB) among cirrhotic patients with and without OVB.
DISCUSSION
This Taiwanese study provides recent data on the cumulative incidence of OVB among cirrhotic patients, which may better reflect current conditions seen in clinical practice. We used a Taiwanese, population-based database to derive important and reliable information on the predictive factors of OVB, as well as the exact cumulative incidence of OVB among cirrhotic patients (computed using competing risk analysis). Among all the predictive factors identified, we found that cirrhotic patients with a history of OVB have a fourfold increased risk of future OVB.
The annual incidence of the first OVB has been estimated to be around 5%–10% among non-selected cirrhotic patients without a history of previous bleeding.(2-4) The cumulative incidence of the first OVB episode in the present study (11.3% in three years) is similar to that in a previous study by Acharya et al.(13) In their study, the cumulative incidence of recurrent OVB was 12.7 per 100 patients/year among cirrhotic patients.(13) This suggests that recent medical progress did not result in any improvement in the prevention of first or recurrent OVB among cirrhotic patients. However, three decades ago, the six-week mortality rate was 42% among cirrhotic patients with OVB; of these, 60% of the early deaths and 40% of the late deaths were attributable to OVB.(14) In recent studies, the reported 4–6-week mortality among cirrhotic patients with OVB was about 10%–20%.(15-17) In the present study, the six-week mortality was 17.1%. Therefore, although medical progress did not correlate with a decrease in the occurrence of OVB, it resulted in better survival among cirrhotic patients with OVB.
In the present study, HCC, young age, ascites, alcohol-related disorders, PUB, diabetes mellitus and a history of OVB were factors found to be associated with increased risk of OVB occurrence. These findings are compatible with those of previous studies.(18-20) In both the present and previous studies,(18-20) the occurrence of OVB was found to be much higher among cirrhotic patients with a history of OVB. This finding highlights the importance of the prevention of first-time OVB. Clinical physicians should consider the use of medications for the prevention of OVB early in the treatment of cirrhotic patients, even if they do not have a history of OVB.
The present study was not without limitations. Firstly, although the severity of cirrhosis is commonly measured using the Child-Pugh score or the Mayo Clinic Model of Care for end-stage liver disease, it was not possible to identify certain laboratory data (e.g. prothrombin time, and bilirubin, albumin and creatinine levels) with the ICD-9-CM codes recorded in the database we used. Also, the endoscopic findings on the varices (e.g. size and shape) could not be identified. Secondly, while about 21.0% of the patients in the present study had alcohol-related cirrhosis, the exact aetiology of non-alcoholic liver cirrhosis could not be identified in this population-based study. Hence, the aetiology (e.g. hepatitis B or C infection) could not be analysed to determine if it could be a predictive factor for OVB among cirrhotic patients. However, the aetiology of non-alcohol-related cirrhosis in Taiwan is known to be mostly related to hepatitis B virus infection, as was well-established in a previously published study.(21) Thirdly, since the Taiwan National Health Insurance programme was developed in 1995, any OVB cases that occurred before then would not have been identified in the present study. However, due to the high recurrence of OVB, we believe that the number of patients with OVB before 1995 is very low in the present study. Finally, the NHIRD database used in our study did not have information on the size of the varices, use of prophylactic band ligation and/or use of beta-blockers. This is an essential flaw of the database. Nevertheless, the large sample size obtained from the database could have mitigated any possible bias from the lack of information.
In conclusion, cirrhotic patients without a history of OVB have low risk of OVB. However, after the first incidence of OVB in cirrhotic patients, the risk for recurrence increases fourfold.
ACKNOWLEDGEMENTS
This study is partially based on data from Taiwan’s NHIRD, which was established and maintained by the Department of Health of the National Health Insurance Bureau and the National Health Research Institutes (Reg No.: 101516). The interpretation and conclusions contained herein do not represent those of the Department of Health of the National Health Insurance Bureau or the National Health Research Institutes.


