Abstract
INTRODUCTION
Conventional knowledge holds that the majority of ruptured atherosclerotic plaques causing ST-segment elevation myocardial infarction (STEMI) are found in moderate stenoses that produce < 50% loss of arterial diameter. This study aimed to analyse the culprit lesions in patients who presented with STEMI and underwent primary percutaneous coronary intervention (PPCI) at our institution.
METHODS
Patients who underwent PPCI between June 2008 and August 2010 at our institution were included in the analysis. Quantitative coronary angiography was performed for the culprit lesions immediately after antegrade flow was restored by thrombectomy, low-profile balloon predilatation or guidewire crossing.
RESULTS
A total of 1,021 patients were included in the study. The mean age was 57 ± 12 years and 85.2% were male. Lesion measurement was done after coronary flow was restored by thrombectomy (73.1%), balloon dilatation (24.1%) and following guidewire passage across the lesion (2.8%). Mean minimal luminal diameter was 1.1 ± 0.5 mm, mean reference vessel diameter was 2.8 ± 0.6 mm, mean diameter stenosis was 61 ± 16% and mean lesion length was 16 ± 6 mm. Most (80.2%) of the culprit lesions had diameter stenoses > 50% (p < 0.01). Although balloon angioplasty was performed in 24.1% of the patients, the majority (64.2%) still had diameter stenoses > 50%. High-grade stenoses (> 50%) were more frequently observed in male patients (p = 0.04).
CONCLUSION
Contrary to the existing paradigm, we found that most of the patients with STEMI in our institution had culprit lesions with diameter stenosis > 50%.
INTRODUCTION
The basic underlying pathophysiology in ST-segment elevation myocardial infarction (STEMI) is plaque rupture in an epicardial coronary artery, with resultant thrombosis leading to vessel occlusion.(1) Previous reports have indicated that acute thrombotic occlusion arises predominantly from plaque rupture at the site of coronary stenosis < 50%, without significant flow limitation.(2-4) However, recent studies have shown that severe stenotic lesions are more ‘vulnerable’, as they carry higher chances of subsequent myocardial infarctions.(5-8) This finding accords with observations in patients evaluated for fractional flow reserve (FFR), in that deferral of percutaneous coronary intervention (PCI) for lesions without haemodynamic significance (i.e. FFR > 0.75) has been shown to be associated with low acute myocardial infarction (AMI) and mortality risks (< 1% per year during follow-up over five years).(9) This suggests that patients with nonsignificant coronary artery stenosis have a relatively low event risk.(9) However, it is difficult to ascertain the severity of a pre-existing lesion immediately before a plaque rupture event, even in cases where diagnostic angiography was performed in the distant past, as the culprit lesion may have progressed in severity during the interim period. The aforementioned discrepancies prompted an investigation of the severity of underlying culprit lesion stenoses following restoration of coronary flow in patients who presented with STEMI in our institution.
METHODS
We retrospectively reviewed 1,043 consecutive patients who presented with STEMI and underwent primary angioplasty (or primary PCI [PPCI]) in the National University Hospital, Singapore from 1 June 2008 to 31 August 2010. Patients from all age groups who had a history of chest discomfort within six hours of hospital presentation and/or an electrocardiogram (ECG) demonstrating STEMI (or new onset of left bundle branch block) and an identified culprit lesion that had been successfully opened on primary angioplasty were included in the analysis.(10) The culprit lesion was defined as the lesion that leads to the index admission and upon which the interventional procedure was performed. If multiple lesions were present and underwent intervention during primary angioplasty, the interventionist on duty would decide which lesion was the most likely cause of presentation, based on the ECG at presentation and/or angiographic appearance.
Quantitative coronary angiography (QCA) was performed using the CAAS Quantitative Coronary Analysis software (PIE Medical Imaging, Maastricht, Netherlands) on the Xcelera Workstation (Philips Medical Systems, Veenpluis, Netherlands). QCA was conducted for all successfully treated lesions documented on cineangiography, immediately following the successful restoration of distal blood flow. Lesions were categorised into three groups according to the method of distal blood flow restoration: (a) wiring and thromboaspiration; (b) wiring and balloon dilatation; and (c) wiring only. The use of intracoronary nitroglycerin prior to cineangiography was subject to the individual operator’s discretion and the clinical condition of the patient.
Patients were excluded from the final analysis based on the following criteria: (a) unsuccessful procedure (i.e. failure to cross the lesion); (b) unobtainable or corrupted cineangiography files; and (c) poor quality film that hindered adequate and consistent QCA analysis. The local ethics committee approved the collection of data from our local database and data analysis. Statistical analysis was performed using Predictive Analytics SoftWare version 18.0 (SPSS Inc, Chicago, IL, USA). Binary variables were summarised as counts and percentages, and compared using two-tailed Fisher’s exact test or t-test, as appropriate. Univariate analysis was used to assess the clinical association among multiple factors.
RESULTS
Of the 1,043 patients who were referred to undergo PPCI for STEMI, only 1,021 patients were included in the final analysis. 22 (2.1%) patients were excluded: 4 (0.4%) had a missing or corrupted cineangiography; 11 (1.0%) had unsuccessful PPCI; and 7 (0.7%) had poor quality films that were unsuitable for QCA. The mean age of the 1,021 included patients was 57 ± 12 years and 85.2% were male. The proportion of patients grouped according to the method of distal blood flow restoration was as follows: (a) wiring and thromboaspiration (n = 746, 73.1%); (b) wiring and balloon predilatation (n = 246, 24.1%); and (c) wiring only (n = 29, 2.8%). The baseline characteristics of the study populations are presented in
Table I
Baseline characteristics of the patients included in the analysis (n = 1,021).
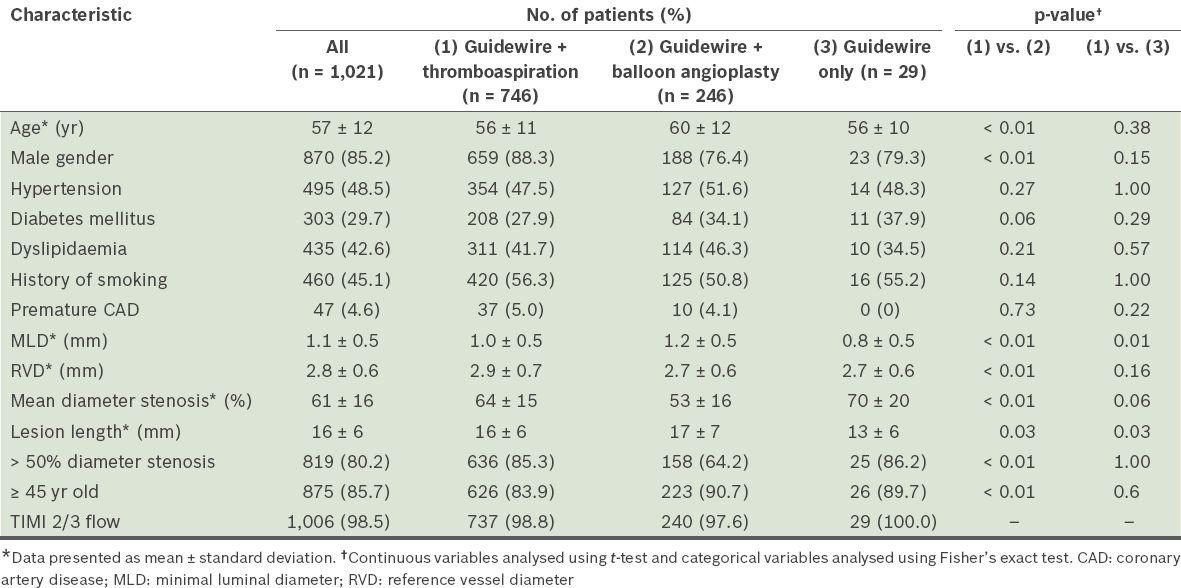
Patients who had balloon predilatation were older (mean age 56 years vs. 60 years, p < 0.01), and had larger minimal luminal diameter, smaller diameter stenosis and longer length of lesion, as compared to the other two groups (all p < 0.05). Lesion length was shortest in patients who had restoration of adequate flow (TIMI-3) by guidewire crossing only (13 mm vs. 16 mm vs. 17 mm, p = 0.03). Overall, the majority of patients (80.2%) had culprit lesion diameter stenoses > 50% following restoration of blood flow. This was more frequently noted in patients who had not undergone balloon predilatation (85.3% vs. 64.2%, p < 0.01). Univariate analysis showed that male patients more frequently had lesion diameter stenoses > 50% (p = 0.04,
Table II
Results of univariate analysis for various factors with respect to lesion severity.
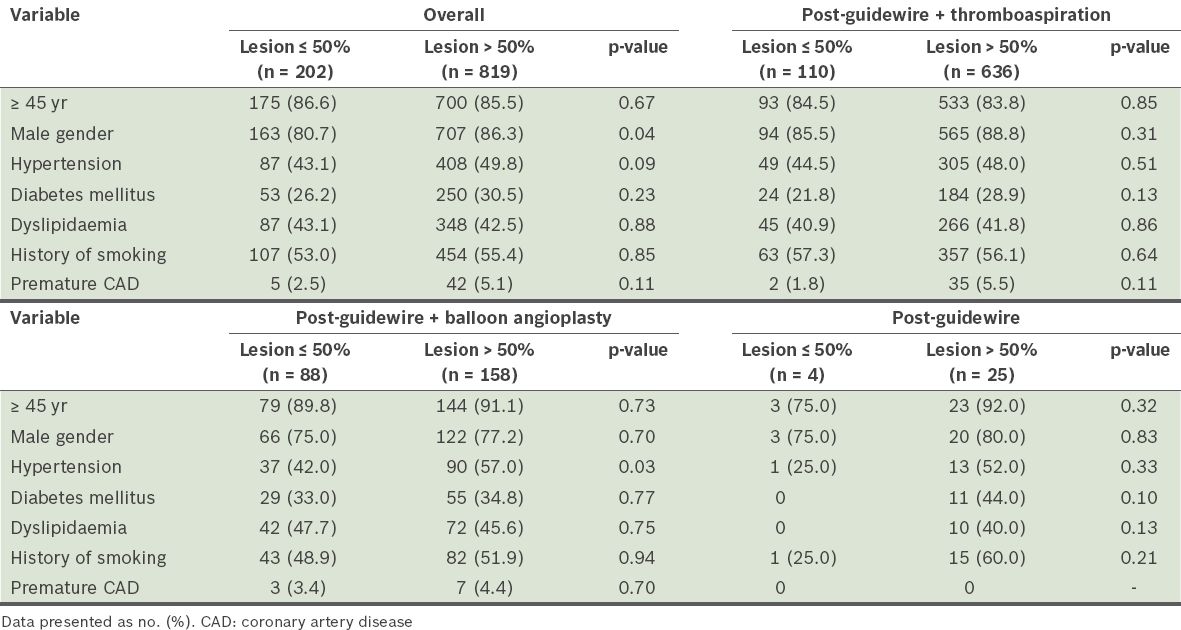
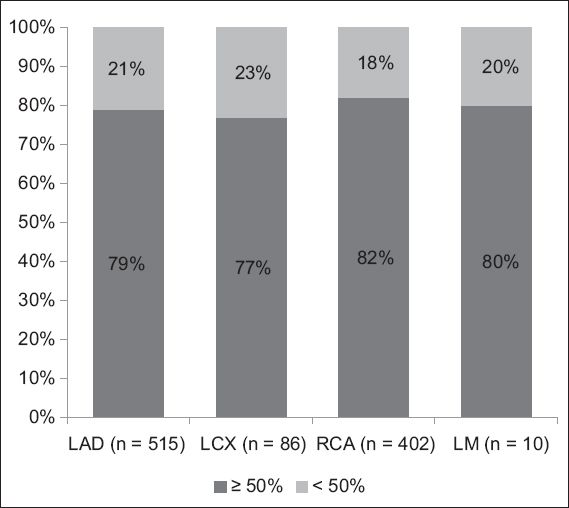
Fig. 1
Bar graph shows the proportion of patients with lesion diameter stenosis ≥ 50% in different culprit coronary arteries. LAD: left anterior descending coronary artery; LCX: left circumflex artery; LM: left main coronary artery; RCA: right coronary artery
DISCUSSION
In the present study, we found that the majority of patients who presented with acute STEMI and subsequently underwent PPCI had an underlying culprit lesion with ≥ 50% diameter stenosis. This was a consistent finding in all native coronary arteries and coronary grafts. It remained true even in the less readily assessed group of patients who underwent predilatation with low-profile angioplasty balloons before the lesions were measured. These findings are in contrast with the conventional belief that the majority of AMI cases occur at coronary arteries with mild lumenal stenoses, as reported in several retrospective studies. For instance, Little et al’s study, which investigated 29 patients with myocardial infarction after a mean duration of 24 months following initial angiography, found that 66% of the patients had a subsequent arterial occlusion with < 50% diameter stenosis on the baseline angiogram.(2) Hackett et al investigated the severity of residual stenosis after successful thrombolysis in 60 consecutive patients at the time of their first AMI and found that residual diameter stenoses < 60% were present in 47% of their patients.(11) Giroud et al investigated 92 patients who had a coronary angiography before and after AMI (median time between first coronary angiography and AMI: 26 [range 1–144] months) and found that 72 (78%) of the segments responsible for future AMI were not significantly stenotic on the baseline angiogram. The authors therefore concluded that AMI was frequently related to a segment that was not the most stenotic or significantly stenosed at the previous angiography.(4) Similarly, in an evaluation of 17 patients who underwent pre- and post-myocardial infarction angiography, Trabulo et al concluded that coronary angiography performed when patients are in a stable condition had a low predictive value for determining the localisation of the arterial segment that would lead to future AMI.(12)
Conversely, some studies have reported that a significant lesion is likely to be the culprit of an acute myocardial event. In a recent retrospective study by Ojio et al, in which angiography was performed within a week prior to AMI, the mean stenosis severity in infarct-related coronary segments was 71%,(13) similar to the findings of the present study. Kotani et al used intravascular ultrasonography (IVUS) to assess culprit and non-culprit plaques within one week of the onset of myocardial infarction and found that culprit lesions had a mean angiographic diameter stenosis of 79%, suggesting that culprit lesions are haemodynamically significant.(14) This finding is in line with the results of the present study. In addition, Kotani et al found that culprit plaques had more positive remodelling, were more likely to be hypoechoic and show more evidence of thrombosis.(14) Prospective studies of large study cohorts have drawn similar conclusions. Alderman et al, in their analysis of 2,161 stenoses (assessed by coronary angiography), found that the coronary artery occlusion rate at five years was strongly correlated to the severity of the initial lesion.(15) Likewise, Nobuyoshi et al reached a similar conclusion in their analysis of 239 prospective patients.(16)
It is also important to note that a number of studies have suggested that nonsignificant lesions have a relatively low per-lesion risk of being the culprit site underlying future cardiac events or associated mortality. For example, the DEFER study, which used FFR to determine whether an intermediate lesion was physiologically significant, showed that physiologically nonsignificant lesions (FFR > 0.75) have a relatively low risk (< 1% per year) of a cardiac event and mortality within the next five years.(9) Abizaid et al, in a prospective IVUS assessment of 300 patients with 357 intermediate coronary artery lesions, revealed that the risk of a future cardiac event was proportional to the severity of the indexed lesion stenosis.(17)
The present study attempted to determine the severity of lesions at the time of primary angioplasty using QCA, similar to a study conducted by Frobert et al.(8) The results of both studies suggest that the underlying culprit lesions in AMI are often haemodynamically significant. However, studies mentioned in the preceding paragraphs have shown contradictory findings. This discordance in findings could be due to the unpredictable time period required for a nonsignificant lesion to progress to a total or subtotal occlusion that results in an AMI. Earlier studies have demonstrated that disease progression takes place in approximately 7%–12% of coronary artery lesions per year based on angiographies taken at yearly intervals.(18-20) Yokoya et al analysed 36 vessels in 36 patients who had four serial coronary angiograms performed at intervals of four months over a one-year period.(20) They found that 39% of the vessels underwent marked disease progression (≥ 15%) within one year; of note, the majority of these vessels (13/14, 92%) had marked progression seen at the final angiography, although the previous two angiographies (taken approximately four and eight months prior) showed no obvious progression (< 5%).(20) These findings could explain our observation that AMIs often result from a significant underlying lesion, which may undergo disease progression on its own over an unknown period of time.
The series by Frobert et al had slightly higher overall lesion stenoses as compared to the present study (~73% vs. 61%). It is noteworthy that none of the patients from Frobert et al’s study received IVUS or thromboaspiration,(8) whereas in our institution, it is a standard practice before primary angioplasty to perform thromboaspiration followed by cineangiography, after the administration of 100–200 mcg of intracoronary nitroglycerin (depending on blood pressure), to demonstrate the underlying lesion. However, some of our patients required balloon angioplasty to restore their blood flow due to the presence of a critical clinical condition or a failure to pass the aspiration catheter across the lesion. This group of patients tended to be female, older and had longer lesion lengths and smaller reference vessel diameters. It is understandable that significantly less lesion stenosis is found on the angiograms of patients who underwent balloon angioplasty (as compared with those who received thromboaspiration and/or guidewire crossing only). However, despite the use of balloon angioplasty before quantitative angiography, the majority of patients in this group still had lesions with diameter stenosis > 50%.
This retrospective study had several limitations. First, 22 (2.1%) patients were not included in the final analysis due to various technical or procedural reasons. Second, it is difficult to maintain a consistent treatment protocol in the setting of an AMI, due to the emergency nature of STEMI and its attendant risk of haemodynamic instability. Thus, the use of intracoronary nitroglycerin prior to cineangiography was at the discretion of the individual operator. Also, as AMI is usually caused by plaque rupture with likely overlying thrombus formation, it is possible that the target lesion appeared to be more severe than it actually was on the angiography. Nevertheless, following careful independent lesion analysis, the majority of lesions were still found to have diameter stenosis > 50%. Finally, there was no standard protocol to guide the thromboaspiration techniques, although most local operators would adopt an aggressive technique whenever feasible. Therefore, it is possible that differing techniques might have impacted the assessment of the luminal severity of the lesions.
In conclusion, we found that the majority of patients who presented with AMI appeared to have an underlying culprit lesion of diameter stenosis > 50%. This debunks the myth that most STEMIs are a result of minor plaque rupture. Further studies with intravascular imaging would be useful to validate the findings of the present study.


