Abstract
INTRODUCTION
The pandemic caused by the H1N1 influenza virus in 2009 resulted in extensive morbidity and mortality worldwide. As the virus was a novel virus, there was limited data available on the clinical effects of the virus on children in Malaysia. We herein describe the clinical characteristics of children hospitalised with H1N1 influenza at a tertiary care centre. We also attempted to identify the risk factors associated with disease severity.
METHODS
In this retrospective study, we compared the characteristics of the children who were admitted to the University of Malaya Medical Centre, Malaysia, for H1N1 influenza during the pandemic with those who were admitted for seasonal influenza in 2002–2007.
RESULTS
Among the 77 children (aged ≤ 12 years) admitted to the centre due to H1N1 influenza from 1 July 2009–30 June 2010, nearly 60.0% were aged < 6 years and 40.3% had an underlying medical condition. The top three underlying medical conditions were bronchial asthma (14.3%), cardiac disease (10.4%) and neurological disorders (11.7%). The risk factors for severe disease were age ≤ 2 years, underlying bronchial asthma and chronic lung disease. Two of the three patients who died had an underlying medical condition. The underlying causes of the deaths were acute respiratory distress syndrome and brain stem encephalitis.
CONCLUSION
The clinical presentation of the children infected with pandemic (H1N1) 2009 influenza virus did not differ significantly from that of children with seasonal influenza. However, there were more complaints of fever, cough and vomiting in the former group.
INTRODUCTION
After the first case of human infection with pandemic (H1N1) 2009 influenza virus was reported in Mexico in April 2009,(1) the infection spread rapidly worldwide.(2,3) By 6 July 2009, there were 94,512 confirmed cases in 123 countries, including 112 cases in Malaysia.(4) The fatality rate of the patients hospitalised with pandemic (H1N1) 2009 influenza was about 10% in the United States and Mexico.(5-7) The most common clinical characteristics reported were fever, rhinorrhoea, cough, vomiting and sore throat.(8-10) Children, the elderly, pregnant women and those with underlying medical conditions were at a higher risk of developing severe complications when infected with this pandemic virus.(11-13)
The global coverage and attention given to the pandemic (H1N1) 2009 influenza was different from that of the typical seasonal influenza. However, differences in the clinical characteristics and spectrum of illness of these two viral infections remain to be determined in the Malaysian population. In the present study, we aimed to describe the clinical characteristics of children with confirmed pandemic (H1N1) 2009 influenza infection who were admitted to a single tertiary care hospital. We also aimed to identify the risk factors for severe disease in our study cohort.
METHODS
This was a retrospective study of paediatric patients with confirmed pandemic (H1N1) 2009 influenza, who were admitted to the University of Malaya Medical Centre (UMMC), Kuala Lumpur, Malaysia, from 1 July 2009 to 30 June 2010. The UMMC is a tertiary care referral hospital. Ethical approval was obtained from the Medical Ethics Committee of the UMMC prior to the commencement of the study.
As the pandemic (H1N1) 2009 influenza virus was a novel virus resulting from a reassortment of its genome, it would be useful to determine its clinical presentation and attempt to identify the risk factors for severe disease compared to normal seasonal influenza. We decided to use a study on laboratory-confirmed influenza cases for comparison, as this was the only known publication that captured detailed clinical and laboratory data.(14) In addition, the hospital settings and laboratory diagnostic methods were similar. Children aged ≤ 12 years who presented with influenza-like illness (ILI) at the time of admission or acquired it during hospitalisation, and had confirmed pandemic (H1N1) 2009 influenza were eligible for inclusion in this study. Demographic data, clinical presentation, laboratory tests performed, treatment administered, duration of hospitalisation and outcomes were retrieved from the hospital’s online reporting system. ILI was defined as a sudden onset of fever (≥ 38ºC) with respiratory tract symptoms and at least one of the following symptoms: muscle ache, headache, extreme fatigue or poor activity. Based on the revised World Health Organization guideline,(15) a patient is defined as having severe or complicated pandemic (H1N1) 2009 influenza if any of the following are present: (a) clinical and/or radiological signs of lower respiratory tract disease (pneumonia), such as dyspnoea, tachypnoea and crepitations; (b) hypoxia (oxygen saturation < 95% by pulse oximetry) requiring supplemental oxygen or mechanical ventilation, and/or an abnormal chest radiograph; (c) central nervous system involvement (e.g. encephalitis and encephalopathy); (d) complications of low blood pressure (e.g. shock and organ failure), myocarditis and/or rhabdomyolysis; (e) invasive secondary bacterial infection based on laboratory testing or clinical signs (e.g. persistent high fever and other symptoms that last beyond three days); (f) admission to the intensive care unit; or (g) death.
The respiratory samples of the children who presented with ILI were obtained via throat swabs, and/or nasopharyngeal or tracheal aspirations (for ventilated patients). The patients included in this study were identified based on the laboratory reports of respiratory samples that tested positive for pandemic (H1N1) 2009 influenza. Detection of pandemic (H1N1) 2009 influenza virus infection was done using real-time reverse transcription polymerase chain reaction, which was carried out in the Virology Laboratory of the Department of Medical Microbiology at the UMMC.
Statistical analysis was performed using SPSS Statistics version 17.0 (SPSS Inc, Chicago, IL, USA). Descriptive data was presented as frequencies (percentages) for discrete variables and medians (ranges) for continuous variables. Multivariate logistic regressions were performed on selected variables to identify the factors associated with severe or complicated disease. Odds ratios with a 95% confidence interval were calculated. A p-value < 0.05 indicated statistical significance.
RESULTS
A total of 77 children aged 22 days to 11.7 years with pandemic (H1N1) 2009 influenza were hospitalised for ILI during the one-year study period (
Table I
Demographics, comorbidities and clinical symptoms of the children who tested positive for pandemic (H1N1) 2009 influenza (n = 77).
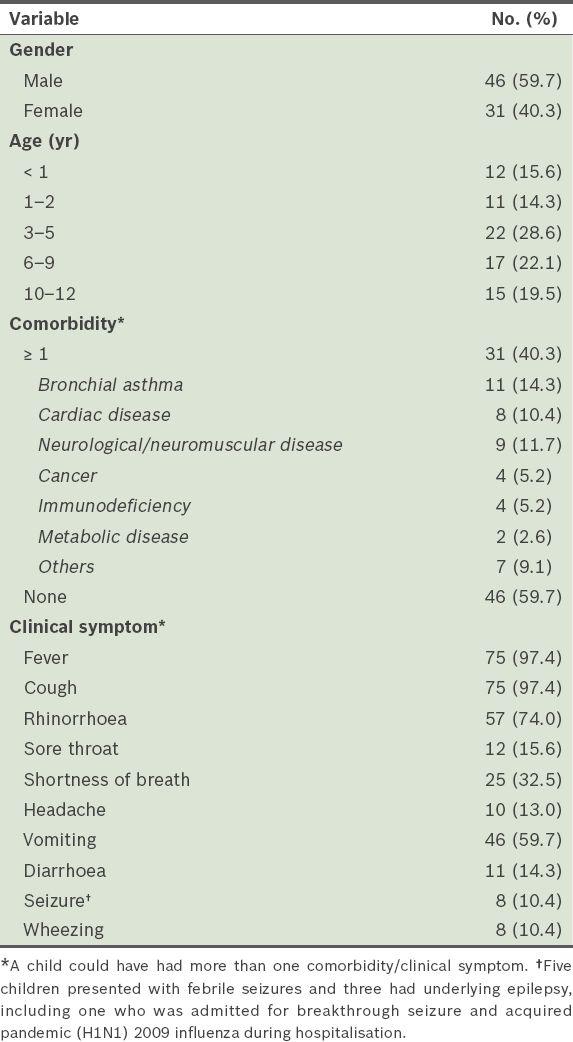
As expected, most of the 77 patients (97.4%) had fever and cough as their main presenting symptoms and about three-quarters (74.0%) had rhinorrhoea. Among the gastrointestinal symptoms observed, vomiting (59.7%) was particularly prominent; only 14.3% had diarrhoea. About a third of the patients (32.5%) had shortness of breath at presentation. The majority (59.7%) of the patients did not have any underlying medical conditions. Bronchial asthma (14.3%), cardiac disease (10.4%) and neurological disorder (11.7%) were the top three comorbid medical conditions among the 77 patients. Five patients had febrile seizures and three had been previously diagnosed with underlying epilepsy. Three patients had nosocomial pandemic (H1N1) 2009 influenza. A nosocomial infection is defined as an infection that was not present at the time of admission, but was acquired after 48 hours of hospitalisation.
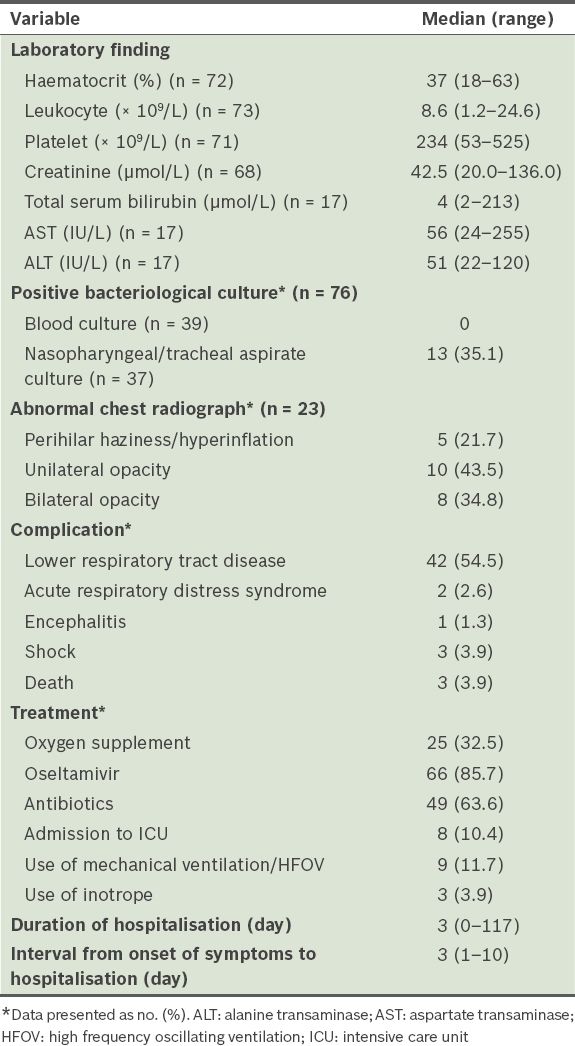
More than half of the patients had complications of the lower respiratory tract; two developed acute respiratory distress syndrome (ARDS), one had encephalitis and three had decompensated shock. The interval between the onset of symptoms and hospitalisation was 3 (range 1–10) days. Most of the children (85.7%, n = 66) received oral oseltamivir, with 24.2% started on it within 48 hours after the onset of illness. Only 8 (10.4%) patients were admitted to the intensive care unit and 3 (4.0%) patients required inotropic support due to shock. 7 (9.1%) patients required mechanical ventilation (two received non-invasive ventilation, while five required intubation) and high frequency oscillating ventilation was used in 2 (2.6%) patients who developed ARDS.
Microbiological cultures were performed on the blood samples and nasopharyngeal/tracheal aspirates of 39 and 37 patients, respectively, out of the 77 patients. Not all patients had a blood and/or nasopharyngeal/tracheal aspirate culture performed. None of the 39 patients had a positive blood culture, while 13 had positive nasopharyngeal/tracheal aspirate cultures. The positive nasopharyngeal/tracheal aspirate cultures were for Streptococcus pneumoniae (n = 4), Haemophilus influenzae (n = 4), Pseudomonas aeruginosa (n = 2), Escherichia coli (n = 2) and methicillin-sensitive Staphylococcus aureus (n = 1). It was not certain whether these positive cultures represented colonisation of the respiratory tract or true infection due to pathogens.
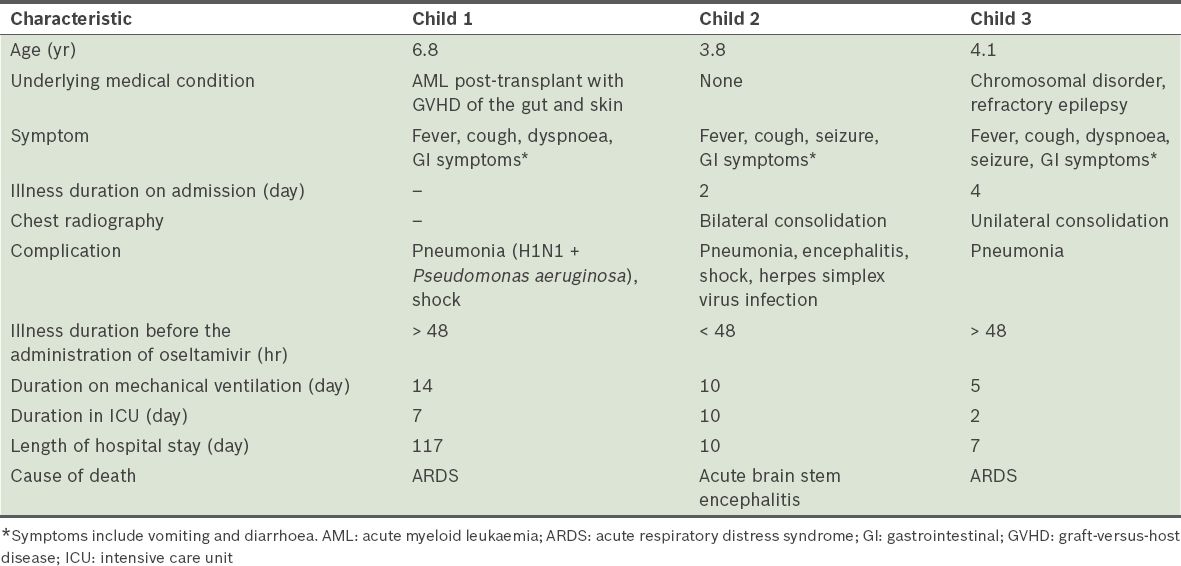
The majority of the patients were considered to have mild disease, with a median hospital stay of three days. One of the patients was hospitalised for 117 days due to graft-versus-host disease after a cord blood transplant, during which she acquired the pandemic (H1N1) 2009 virus infection. The most common cause of death was ARDS and brain stem encephalitis. Among the 77 patients, three patients who had underlying medical conditions died; they were aged between three and seven years (
Table II
Characteristics of the three hospitalised children who died of pandemic (H1N1) 2009 influenza.
The laboratory values, complications and treatment of the hospitalised children with pandemic (H1N1) 2009 influenza are presented in
Table III
Laboratory and radiographic findings, treatment(s) administered, duration of stay and complications among the patients (n = 77).
Results of multivariate logistic regressions showed that the risk factors for severe or complicated pandemic (H1N1) 2009 influenza among children were age ≤ 2 years and the presence of asthma or chronic lung disease (
Table IV
Risk factors for severe or complicated pandemic (H1N1) 2009 influenza virus infection.
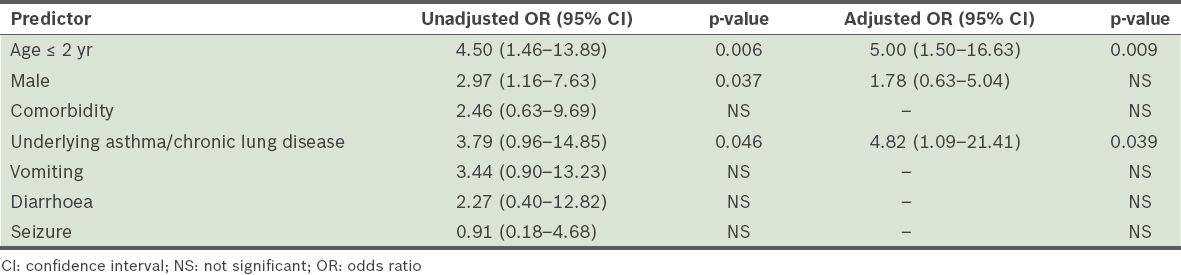
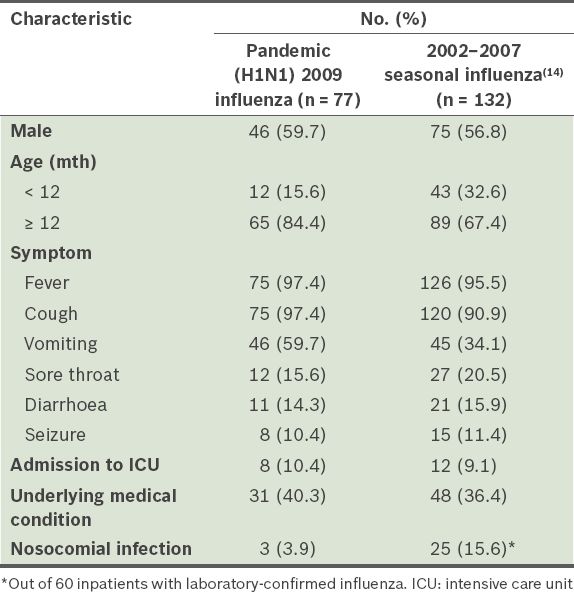
Table V
Comparison of selected characteristics of hospitalised children with pandemic (H1N1) 2009 influenza and those with 2002–2007 seasonal influenza.
DISCUSSION
In the present study, we reported the clinical characteristics of hospitalised children with pandemic (H1N1) 2009 influenza and the risk factors for severe disease. Incidence of the pandemic (H1N1) 2009 influenza peaked in August and declined markedly after September 2009, with an average of two cases detected monthly. Seasonal influenza cases were reported all year round, peaking between November–January and May–July, with an average of four cases monthly.(14) Among hospitalised children in the present study, those of age 3–5 years formed the largest group of children who were infected.
Although the haematological and biochemical parameters of the patients varied widely, they did not differ significantly from the normal limits. This could be explained partly by the presence of underlying diseases or secondary bacterial infections in some of the children. Similar to figures reported in several other studies, 85.7% of the children in the present study were given oseltamivir.(8,16-19) The pandemic (H1N1) 2009 influenza virus caused severe illness, including ARDS and encephalitis, and resulted in 10.4% of the children being admitted to the intensive care unit. Although underlying medical conditions were common among the hospitalised children, we also identified cases of severe illness due to the H1N1 virus infection among children who were previously healthy.
Based on the judgment of the admitting medical officer, only 46 of the 77 patients in the present study had chest radiography performed at presentation. Among these 46 patients, 35 films were formally reported by a radiologist. About one-third (34.5%) of the radiographs were normal, while the remaining radiographs had either unilateral opacity (28.6%), bilateral opacities (22.9%) or perihilar haziness (14.3%), consistent with interstitial pneumonia. However, based on the radiographs, it was not possible to conclusively attribute the lung changes to a viral or bacterial aetiology.
The results of the present study corroborated the findings of several other studies on the association between asthma and severe disease in children and adults.(11,20,21) Thus, it is likely that efforts to achieve good control of bronchial asthma can help to minimise the complications and severity of the pandemic (H1N1) 2009 virus infection. Underlying medical conditions other than asthma or chronic lung disease were not found to be significant risk factors for disease severity in the present study. This is possibly due to the small sample size of paediatric patients in the present study or the fact that children with pre-existing conditions are more likely to have sought prompt medical consultation as compared to healthy children.
Although the symptoms of patients with pandemic (H1N1) 2009 influenza resemble those of patients with seasonal influenza,(17,22-24) with most of the cases being categorised as mild, we found that there were more complaints of fever, cough and vomiting among children with pandemic (H1N1) 2009 influenza in the present study. The incidence of medical symptoms and complaints from this viral infection may have been underreported, as children with mild cases of infection were not admitted to this centre under the directive of the Malaysian Ministry of Health. This directive was issued as part of the ministry’s mitigation strategy to prioritise healthcare resources for moderate to severe cases due to the increase in the number of local transmissions during the viral outbreak.
When we compared our study cohort with children who were admitted to the same tertiary care centre for seasonal influenza in 2002–2007, we found that there was a higher incidence of nosocomial infection among the latter. The lower incidence of nosocomial transmission among the children with pandemic (H1N1) 2009 influenza was expected, as during this time, there was intense daily coverage by the local media on the number of cases and deaths related to this infection, as well as campaigns to raise public awareness on measures to prevent disease transmission. The mitigation strategy employed by the hospitals may have also reduced the spread of this disease. In contrast, the spread of seasonal influenza in 2002–2007 did not receive similar aggressive coverage by the media. In addition, resource allocation for seasonal influenza was virtually non-existent compared to that for pandemic (H1N1) 2009 influenza.
Although the duration of hospitalisation of the children infected with pandemic (H1N1) 2009 influenza and those infected by seasonal influenza in 2002–2007 could not be directly compared in the present study, a report by Bagdure et al(17) showed a longer hospitalisation duration among children with seasonal influenza. We speculate that the higher rate of antiviral therapy administered to children with pandemic (H1N1) 2009 influenza could be the result of its elevation to pandemic status by the World Health Organization on 11 June 2009. Compared with the mortality rate among patients with pandemic (H1N1) 2009 influenza, deaths from seasonal influenza are usually rare. This is reflected in the low mortality rate observed in the 2002–2007 seasonal influenza study.(14)
In the present study, secondary infection or bacterial coinfection was seen in a number of the children admitted with pandemic (H1N1) 2009 influenza. While preventive vaccines against pulmonary infection due to Pseudomonas aeruginosa, Escherichia coli and/or Staphylococcus aureus have yet to be developed, vaccines against Streptococcus pneumoniae and Haemophilus influenzae are available. There is a strong case to vaccinate all children against Streptococcus pneumoniae and Haemophilus influenzae as these bacterial infections can cause severe disease and even lead to the deaths of those already infected with the influenza virus.
The present study was not without limitations. Firstly, there was missing or incomplete data as the data was collected retrospectively. This may have diminished the power of our analysis. Secondly, as the present study only included children with confirmed pandemic (H1N1) 2009 influenza in a single centre, caution should be exercised when generalising the results of this study to the Malaysian population. Thirdly, confirmatory laboratory tests were only carried out on hospitalised children who had severe or complicated diseases. In other words, information on the majority of the children (who had mild to moderate disease) would not have been captured, resulting in the underreporting of these cases. Finally, the comparison of our study cohort with children who were admitted to the same tertiary care centre for seasonal influenza in 2002–2007 may not have been fair, as these two cohorts of patients may not have had comparable characteristics. Furthermore, data on the two cohorts were captured during different time periods and were thus under the influence of different compounding factors.
To conclude, it is our hope that the findings of the present study will contribute to current investigations that aim to improve the understanding of the clinical spectrum of the pandemic (H1N1) 2009 influenza among Malaysian children, so that better preventive measures, in particular annual immunisation, can be taken to minimise complications and mortality in future outbreaks.


