Abstract
Cardiovascular and noncardiovascular conditions are commonly encountered in the emergency department. While the majority of patients have underlying cardiovascular aetiologies, such as acute myocardial infarction, congestive heart failure, aortic dissection and pulmonary embolism, a small subset of patients have underlying noncardiovascular conditions, although they present with similar symptoms of chest pain, dyspnoea, cough, haemoptysis and haematemesis. This article aims to describe the imaging findings in common noncardiovascular conditions of the chest that are frequently encountered in the emergency department, with a review of the existing literature.
INTRODUCTION
Thoracic emergencies are mainly diagnosed and differentiated through clinical history, physical examination, electrocardiography and laboratory investigations. For a substantial proportion of patients presenting to the emergency department (ED), a specific diagnosis remains elusive and imaging plays an important role in the assessment of patients with cardiac and noncardiac chest conditions. This article aims to discuss the role of radiography and computed tomography (CT) of the chest in evaluating noncardiovascular thoracic diseases encountered in the ED. It illustrates the spectrum of noncardiovascular thoracic emergency conditions and reviews specific imaging features of conditions that are commonly encountered. Although clinical presentations may vary, there are many similarities in presenting symptoms, signs and clinical history across different conditions. Based on the dominant clinical features, acute conditions can be divided into seven categories (
Table I
Common clinical presentations and noncardiovascular diseases encountered in the emergency department that require radiological investigations.

ROLE OF IMAGING
Chest pain
Chest pain is the most common, specific and principal reason for ED visits by adults.(1) Cardiac causes are the most alarming, followed by pulmonary, musculoskeletal and oesophageal causes. Imaging does not play a major role in diagnosing musculoskeletal aetiologies. However, chest radiography and CT are useful for evaluating chest pains of pleuropulmonary origin, such as pneumothorax, pneumohaemothorax and pneumomediastinum.
Erect chest radiography is sufficient to demonstrate pneumothorax and pneumomediastinum. CT is performed to identify the underlying aetiology, so that proper treatment can be planned to prevent a recurrence. In patients with pneumothorax, CT can reveal underlying blebs, emphysema, cystic lung disease, infection or interstitial lung disease (
Fig. 1
Large spontaneous pneumothorax in a 40-year-old woman with lymphangioleiomyomatosis. (a) Frontal chest radiograph shows a large right-sided pneumothorax with collapsed lung at the hilum (white arrow). (b) Axial CT image shows numerous cysts in both lungs (white arrows), with a chest tube for drainage of the pneumothorax (black arrow).
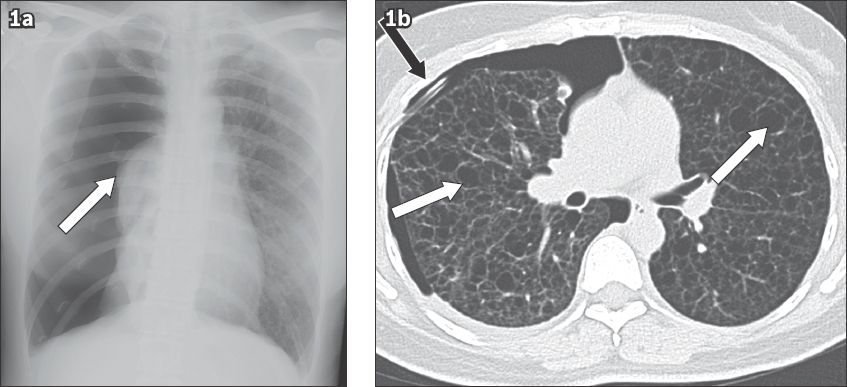
Fig. 2
Spontaneous haemopneumothorax in a 39-year-old man presenting to the emergency department with chest pain and giddiness. (a) Frontal chest radiograph shows a large amount of left-sided pleural fluid, without evidence of pneumothorax. (b) Axial CT image shows an air-fluid level (white arrow) in the left hemithorax with a large complex fluid component. The aerated right lung shows subpleural blebs (black arrows). The drained fluid was dark-coloured, thick and bloodstained. The haemopneumothorax was probably secondary to bleeding in spontaneous pneumothorax from a ruptured vascularised bleb or due to vascular injury.
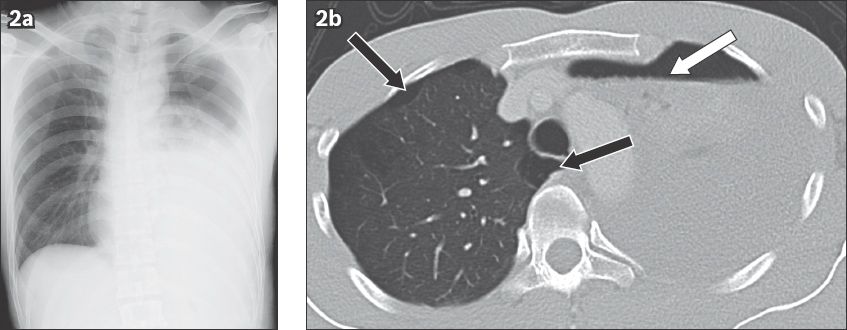
Fig. 3
Spontaneous pneumomediastinum in a 17-year-old boy presenting to the emergency department with sudden onset of excruciating chest pain. (a) Frontal chest radiograph and (b) coronal CT image show paracardiac air (white arrowheads), continuous diaphragm sign (white arrows), and supraclavicular emphysema (black arrows) and emphysema in the superior mediastinum (black arrowhead).
Oesophageal inflammation and gastro-oesophageal reflux disease can lead to chest pain. A moderate-to-large hiatal hernia can be visualised on chest radiography, but it may or may not be the cause of the pain. Life-threatening cardiogenic pain needs to be excluded before the diagnosis of gastro-oesophageal reflux disease can be considered. Infrequently, severe oesophagitis (
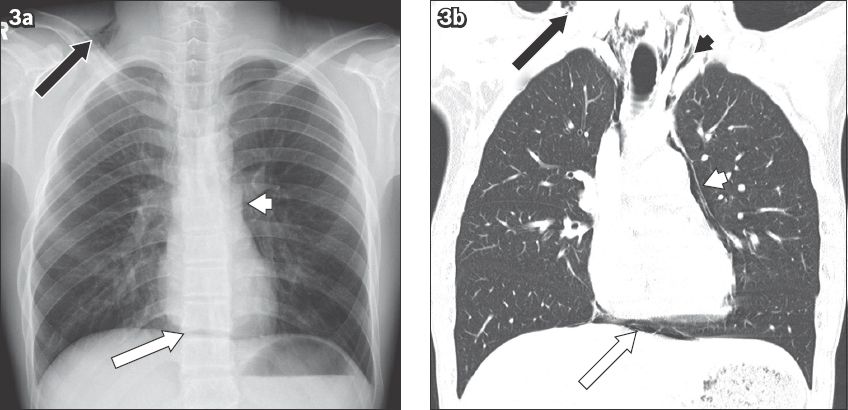
Fig. 4
Severe ulcerative oesophagitis in a 70-year-old man with renal failure. (a) Frontal chest radiograph shows vertical linear lucency (black arrows) in the midline, representing the dilated oesophagus. (b) Axial CT image of the upper oesophagus shows diffuse thickening of the oesophageal wall (white arrow) and an overhanging mucosa (black arrow), creating a ‘double lumen’, which is associated with fat stranding in the mediastinum (white arrowhead).
Infection
Chest radiography remains the initial investigation of choice for all patients presenting with signs of pneumonia at the ED. Frontal radiography of the chest provides useful information about the extent and severity of the pneumonia and associated pleural effusions. Chest radiography followed by CT can help in identifying the source and aetiology of the pneumonia in a small fraction of patients. In patients with Klebsiella pneumonia, a classical buldging fissure sign (
Fig. 5
Klebsiella pneumonia in a 35-year-old man presenting to the emergency department with high-grade fever, rigors, cough and high total white blood cell count. (a) Frontal chest radiograph shows a large, dense homogeneous opacity in the right upper hemithorax, with a sagging inferior border (black arrow). (b) Corresponding coronal CT image shows necrotising consolidation (black arrow) in the right upper lobe with the bulging fissure sign (white arrow). (c) Follow-up chest radiograph after treatment shows residual scarring in the right upper lung (black arrow).
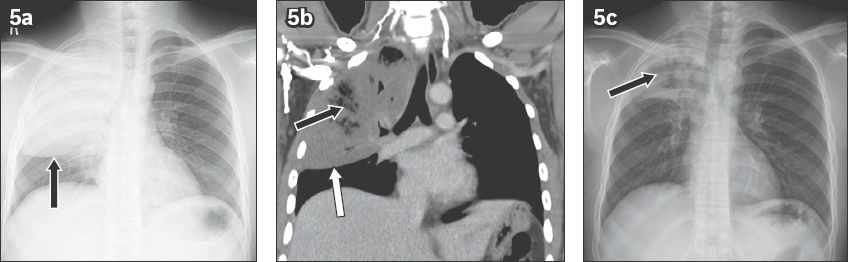
Fig. 6
Septic pulmonary emboli in a 62-year-old man being treated for liver abscess. (a) Frontal chest radiograph shows multiple peripheral wedge-shaped opacities. (b) Coronal CT image shows peripheral pulmonary opacities with cavitations (white arrows). (c) Coronal CT image in soft tissue window shows a left hepatic abscess (black arrow) and cavitary lesions (white arrows).
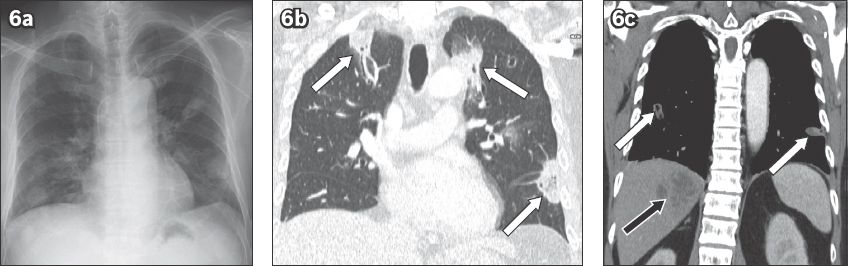
Fig. 7
Amoebic hepatopulmonary abscess in a 55-year-old man presenting to the emergency department with cough and fever. (a) Lateral chest radiograph shows an ill-defined opacity in the posterior lower lung (arrow). (b) Sagittal CT image shows transpleural rupture of the liver abscess into the lower lobe of the left lung (arrow). Only amoebic liver abscesses are known to follow this route in the endemic zone.
Dyspnoea
Most patients who present to the ED with dyspnoea as the predominant symptom have an underlying cardiac disease, but a significant proportion of them have an underlying pulmonary pathology. Chest radiography is a useful tool to differentiate between these two subsets of patients. Imaging also plays an important role in diagnosing the most common pulmonary causes of dyspnoea – acute lung collapse (
Fig. 8
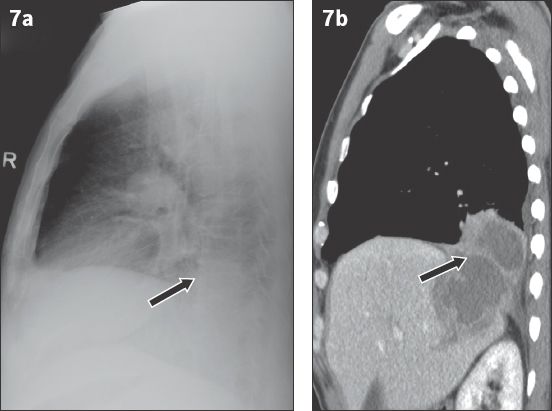
Acute left lung collapse due to an endobronchial carcinoid tumour in the left main bronchus in a 51-year-old woman presenting to the emergency department with sudden onset dyspnoea. (a) Frontal chest radiograph shows an opaque left hemithorax with a mediastinum that has shifted to the left. (b) Coronal CT image shows an endobronchial mass (arrow) with collapse of the left lung (*).
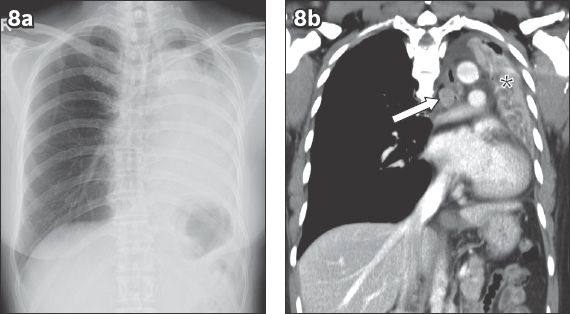
Fig. 9
Bronchial tear in a 34-year-old woman with acute exacerbation of steroid-resistant asthma. Axial CT image shows a defect in the medial wall of the left main bronchus (arrow) with small pneumomediastinum.
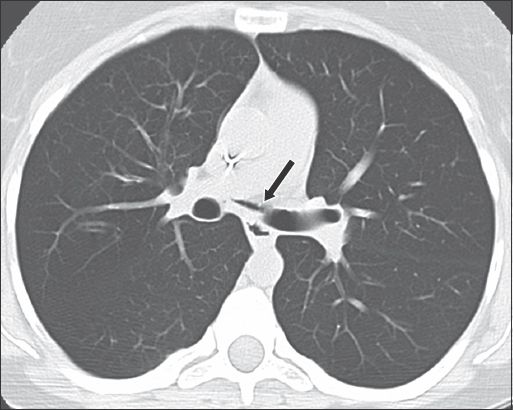
Acute interstitial pneumonitis, a rare acute condition that affects an otherwise normal lung, is characterised by progressive dyspnoea and multifocal pulmonary opacities.(11) Diffuse alveolar damage is the histological hallmark of this condition.(12) Acute interstitial pneumonitis worsens rapidly and has a mortality rate of more than 50%.(12) Radiological findings are similar to acute respiratory distress syndrome and vary according to the phase of the disease.(13) CT is considered the gold standard in characterising ILD. The presence of new ground-glass opacities in a background of honeycombing suggests an acute exacerbation of ILD or superimposed infection.(14) The mortality rate is more than 80% within one month of acute exacerbation.(14)
Hypovolaemic shock
Pleuropulmonary bleeding can be life-threatening, leading to hypovolaemic shock. In patients with haemoptysis, haemothorax can remain clinically undiagnosed or underestimated even when pulmonary sources of bleeding are suspected. Causes of nontraumatic haemothorax include spontaneous pneumothorax, coagulopathy, malignancy and, rarely, pulmonary arteriovenous malformation (PAVM). Imaging, particularly CT, can demonstrate the presence of blood in the pleural cavity, which appears as high-density fluid. Contrast-enhanced CT can identify the source of bleeding (
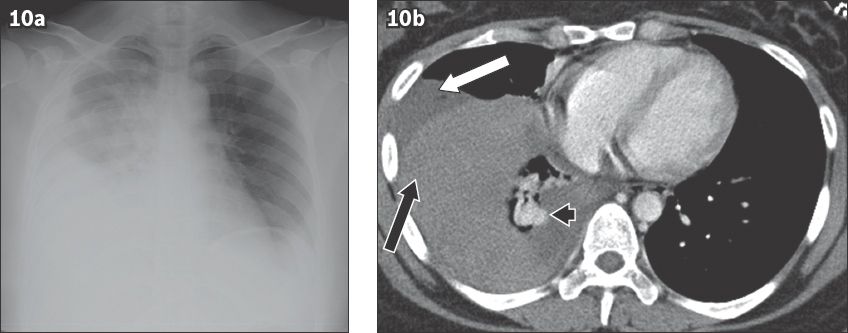
Fig. 10
Spontaneous haemothorax from an actively bleeding pulmonary arteriovenous malformation in a 26-year-old woman presenting to the emergency department with hypovolaemic shock, which was preceded by an episode of sharp right-sided chest pain. (a) Frontal chest radiograph shows a large amount of right-sided pleural fluid. (b) Axial CT image shows high-density dependent fluid (black arrow) and low-density nondependent fluid (white arrow) with an enhancing tortuous vascular malformation (arrowhead).
Haemoptysis
Life-threatening haemoptysis in patients presenting to the ED could be due to malignancy, bronchiectasis, tuberculosis or pulmonary haemorrhage syndrome. The parenchymal changes associated with most of these cases can be identified on chest radiography. CT can identify lesions that are invisible on radiographs and provide more details about underlying pathology, such as demonstrating hypertrophied bronchial arteries, pseudoaneurysms and PAVMs. CT can also provide a road map for bronchoscopy or radiological intervention.(16,17) In a majority of cases (90%), bronchial arteries are the source of bleeding, while in a small number of cases (10%), the culprit vessel originates from pulmonary circulation. Rare entities like Rasmussen’s aneurysm, a mycotic aneurysm of the pulmonary artery (
Fig. 11
Massive haemoptysis in a 54-year-old man. (a) Frontal chest radiograph on presentation to the emergency department shows right upper lung consolidation and a thick-walled cavity in the left upper lung (black arrow). (b) Frontal chest radiograph taken two hours later shows increased fluid level in the cavity (black arrow). (c) Axial CT image shows a small enhancing Rasmussen’s aneurysm (white arrow) in the wall of the cavity. The fluid in the cavity is hyperdense, which suggests blood.
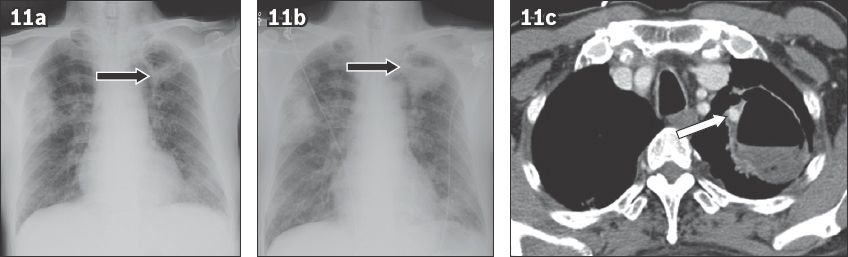
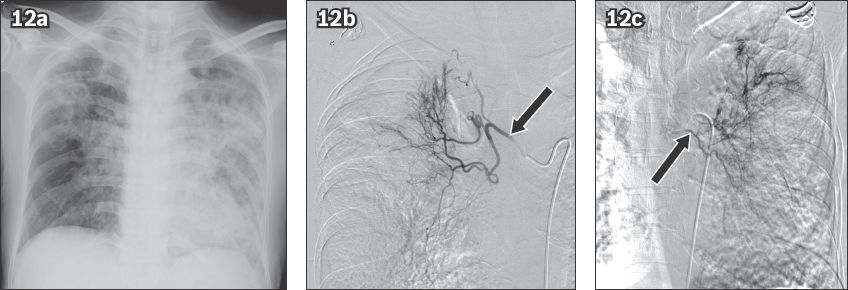
Fig. 12
Haemoptysis in a 51-year-old woman with pulmonary tuberculosis. (a) Frontal chest radiograph shows consolidation in both lungs. Angiograms of (b) the right bronchial artery (arrow) shows tortuous hypertrophied vessels in the right upper zone and (c) the left bronchial artery injection (arrow) also shows multiple tortuous hypertrophied branches that were embolised using polyvinyl alcohol particles.
Diffuse pulmonary haemorrhage or diffuse alveolar haemorrhage (DAH) can present with life-threatening haemoptysis. DAH is also associated with pulmonary infiltrates, dyspnoea and chronic anaemia.(20,21) Its diagnosis is supported by the finding of haemorrhagic fluid in bronchoalveolar lavage. DAH is a clinical syndrome and includes a number of conditions, which are broadly divided into two categories: DAH with and without vasculitis. In clinical practice, the three most common causes of DAH are vasculitis, collagen vascular diseases and drugs (
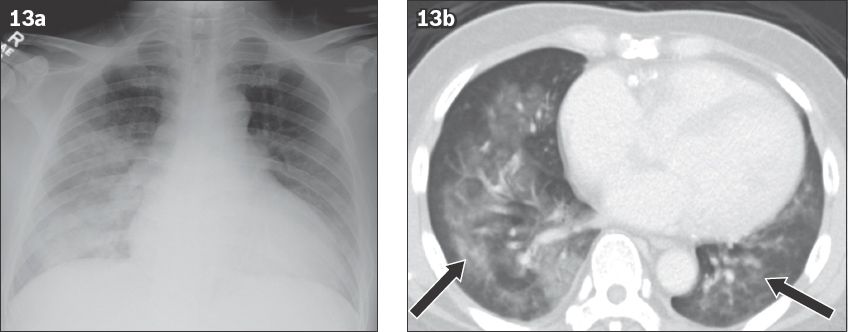
Fig. 13
Diffuse alveolar haemorrhage in a 61-year-old man on warfarin, presenting to the emergency department with haemoptysis. (a) Frontal chest radiograph shows asymmetric ground-glass opacities in the bilateral lower lungs. (b) Axial CT image shows ground-glass opacities (arrows) in both lungs, representing haemorrhage.
Haematemesis
Haematemesis is the result of acute upper gastrointestinal bleeding, commonly due to peptic ulcer disease, varices and oesophagitis.(22) The role of imaging is limited and endoscopy remains the investigation of choice. Boerhaave syndrome is a rare but historically and clinically important condition that causes haemoptysis and can be suspected on imaging.(23) In Boerhaave syndrome, a tear occurs in the posterior wall of the lower oesophagus, usually in the left wall. Classically, the patient has a history of overindulgence in food and drink, with violent vomiting and associated haemoptysis. Delayed diagnosis results in subcutaneous emphysema, chest pain and cardiovascular collapse. Chest radiography typically shows large pleural effusions, pneumomediastinum, pneumothorax and chest wall emphysema (
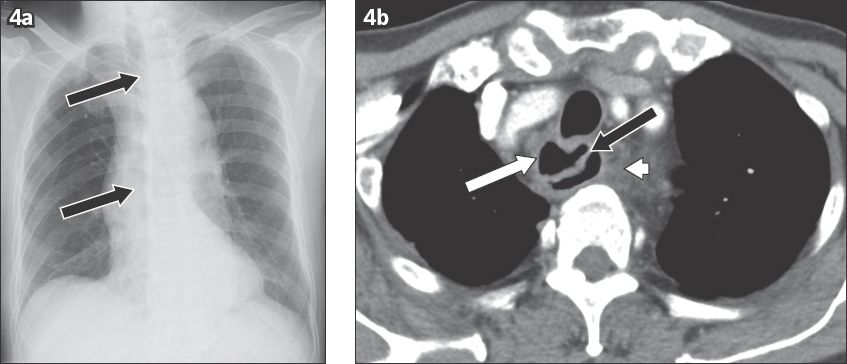
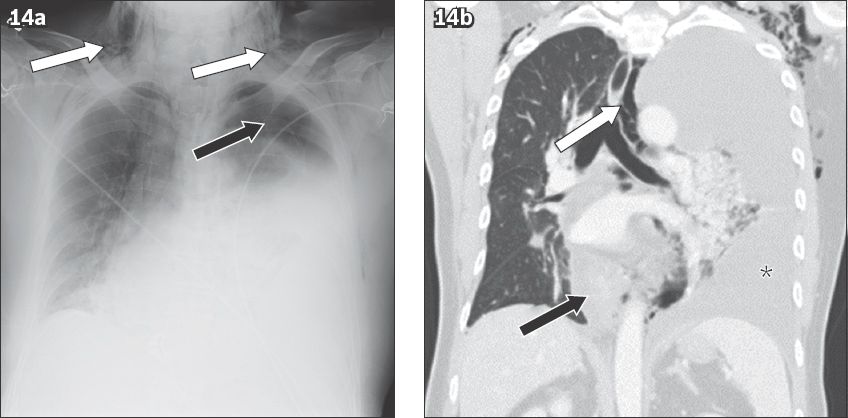
Fig. 14
Oesophageal tear in a 51-year-old man presenting to the emergency department with haematemesis preceded by retching. (a) Frontal chest radiograph shows a large left pleural effusion, left pneumothorax (black arrow) and chest wall emphysema (white arrows). (b) Coronal CT image shows a thickened lower oesophagus due to haematoma (black arrow), a large left pleural effusion (*) and pneumomediastinum (white arrow).
Foreign body aspiration
A foreign body lodged in the tracheobronchial tree can be life-threatening due to airway obstruction (
Fig. 15
Coin aspiration by a 51-year-old man. (a) Frontal chest radiograph shows the metallic foreign body projected over the right hilum (black arrow). (b) Axial CT image shows the coin lodged in the bronchus intermedius (white arrow).
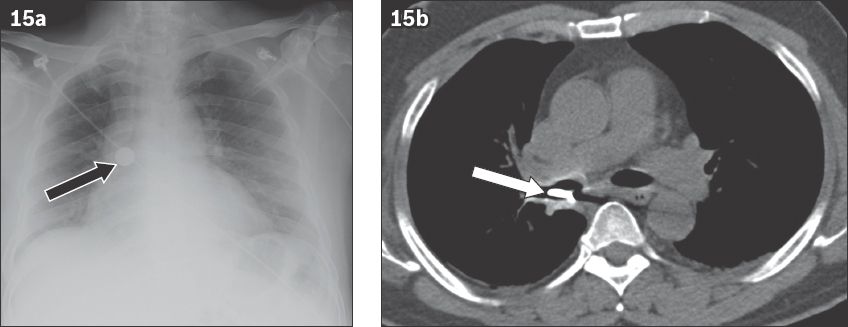
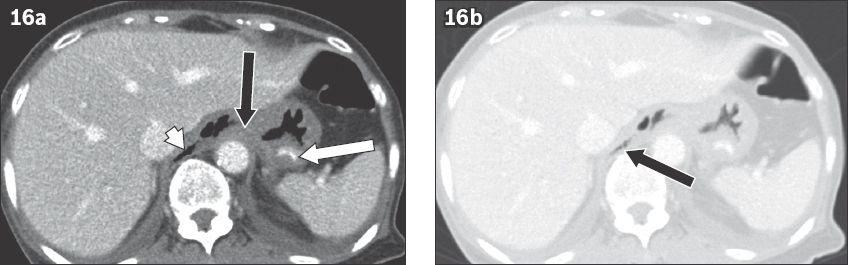
Fig. 16
Gastro-oesophageal perforation in a 28-year-old man after ingesting a chicken bone. (a) Axial CT image shows a curvilinear foreign body lodged in the gastric fundus (white arrow), the perforation site at the gastro-oesophageal junction (black arrow) and free air (white arrowhead). (b) Axial CT image at the same level with a wider window setting shows pneumomediastinum (black arrow).
CONCLUSION
Chest radiography and CT are useful tools for examining patients with emergency chest conditions. Normal chest radiography helps to rule out noncardiovascular emergencies. Abnormal or equivocal radiography findings can be due to various pathologies affecting the thoracic structures. Noncardiovascular acute conditions are less common and require a high index of suspicion on the part of the radiologist. Early diagnosis prevents a delay in definitive management in an often overcrowded ED.


