INTRODUCTION
Primary aldosteronism (PA) affects 5%–10% of all patients with hypertension. About half of these patients have unilateral disease that can be cured with adrenalectomy.(1,2) Although adrenal vein sampling (AVS) is crucial for identifying unilateral PA,(3) it is operator dependent and technically difficult. While excellent AVS rates have been reported in high-volume specialised centres,(4,5) most other institutions worldwide have cannulation rates as low as 31%–61%,(6,7) leading to missed opportunities to cure. While surgery ameliorates the risk of cardiovascular disease, atrial fibrillation and death, patients treated with medications remain at an increased risk of complications,(8,9) further highlighting the importance of identifying unilateral PA.
There are some alternatives to AVS, such as functional imaging using 11C-metomidate positron emission tomography/computed tomography. It is a non-invasive alternative(10) but has not been validated in large studies and is not widely available.(11) Use of the computed tomography (CT) approach alone to identify unilateral disease led to similar blood pressure improvements after surgery compared to AVS in an randomised control trial.(12) However, more patients in the CT arm failed to be cured of PA.(13) Furthermore, CT diagnosis has been reported to lead to false conclusions in 37.8% of patients.(14,15) Ultimately, having an effective AVS service is essential for optimising treatment of PA.
Several centres have utilised novel methods, such as the rapid cortisol assay,(16-18) to improve AVS success, with mixed results.(19-21) At our institution, we reviewed our AVS practice and incorporated several strategies in our programme to improve AVS rates. We herein report the success rates of our AVS programme, which was initiated in 2015. We also suggest practical measures that can be adopted by other low-volume centres worldwide to achieve similar success.
METHODS
We conducted a retrospective study of all patients who underwent AVS in our referral centre, Changi General Hospital, Singapore, from 1998 to 2019. AVS success rates before and after the implementation of an AVS programme in 2015 were assessed. Patient medical records were reviewed, and data on demographics, comorbidities, biochemistry, plasma renin activity (PRA) and plasma aldosterone concentration (PAC) was collected. Before hormonal tests, antihypertensive medications that interfere with the renin-angiotensin-aldosterone system were discontinued for at least two weeks in most patients, and potassium-sparing diuretics were stopped at least six weeks in all patients. Hypokalaemia was corrected with potassium supplementation, targeting a serum potassium level ≥ 3.5 mmol/L. Patients underwent a seated saline infusion test, with a post-saline PAC level ≥ 140 pmol/L confirming the diagnosis of PA. PRA and PAC were determined in all patients based on previous methodology.(22,23) The lower limit of PRA was 0.15 ng/mL per hour before 2016 and 0.6 ng/mL per hour from 2017 onwards.
The study was approved by the local ethics committee, which waived the requirement for informed consent from patients seen before November 2017, while consent was obtained from all patients after November 2017. All patients fulfilled the diagnostic criteria for PA according to the Endocrine Society guidelines,(2) as we have previously reported.(22) All patients underwent thin-section CT. Unilateral adenoma was defined as the presence of a unilateral nodule with a diameter ≥ 8 mm, with a normal contralateral adrenal gland.
Sequential AVS under continuous corticotropin stimulation has been performed since 1998, with corticotropin infusion (50 mcg per hour) started at least 30 minutes before the start of the procedure. Adrenal veins were sequentially cannulated via a right femoral vein vascular sheath, and the catheter tip position was verified with an injection of non-ionic contrast medium. Blood was sampled from both adrenal veins and the infra-renal inferior vena cava (IVC). In 2015, potential pitfalls with previous AVS were identified, and an AVS programme was implemented and several measures were put in place: (1) a dedicated interventional radiologist, assigned to perform or supervise all the procedures; (2) routine contrast-enhanced CT imaging, used for assessment of adrenal vein anatomy prior to AVS; (3) use of at least a 4-French (Fr) Cobra catheter for the right adrenal vein cannulation (routinely attempted first) and 4-Fr Simmons 2 glide catheter for the left adrenal vein cannulation, with a check venogram to confirm the position; (4) routine creation of side holes close to the catheter tips; (5) gentle aspiration of blood to reduce the risk of sampled veins collapsing during the procedure; (6) two right adrenal vein specimens during each AVS (initiated from 2016), involving repositioning of the catheter with a recheck venogram; (7) ‘rapid’ cortisol reporting obtained by dispatching AVS serum cortisol to the laboratory and immediate analysis at neat and 1:20 dilution; (8) keeping the femoral vein sheath in situ after the procedure, with a view to repeat AVS immediately after if cortisol results indicate failure of cannulation; (9) computerised labels for each sample: ‘right #1 adrenal vein’, ‘right #2 adrenal vein’, ‘left adrenal vein’ and ‘IVC’; and (10) collaboration with the laboratory to ensure that absolute values were reported for all cortisol and aldosterone samples.
We evaluated for improvement in the rate of successful bilateral cannulation before and after implementation of the AVS programme. We also assessed the selectivity index (SI), proportion of patients identified with unilateral PA and concordance of AVS with CT. Adrenal vein cannulation was deemed successful if the SI was > 3, as recommended by an expert consensus.(24) The SI was calculated by measuring the ratio of cortisol levels in the adrenal vein to that in the IVC. After 2016, multiple samples were taken from the right adrenal vein, and we assessed the utility of this intervention. Lateralisation ratio was calculated using the aldosterone-to-cortisol ratio between both adrenal veins. A lateralisation ratio ≥ 4 was consistent with unilateral PA, while a ratio ≤ 2 was consistent with bilateral PA. In patients with ratios between 2 and 4, AVS results were discussed at a multidisciplinary meeting for the final decision. Because absolute values of cortisol and aldosterone are required for interpretation of AVS results, failure of the laboratory to provide absolute values was noted and AVS was considered unsuccessful.
Statistical analyses were performed using IBM SPSS Statistics 21.0 (IBM Corp, Armonk, NY, USA). Continuous variables were expressed as median (interquartile range [IQR]) and categorical variables were expressed as number and percentage. Continuous variables were analysed using the Mann-Whitney U test and categorical variables were analysed using Fisher’s exact test. All tests were two-tailed, with differences considered significant when p < 0.05.
RESULTS
A total of 104 AVS procedures were performed in 96 patients from 1998 to 2019 in a single tertiary centre (
Table I
Baseline characteristics of patients before and after programme implementation (n = 96).
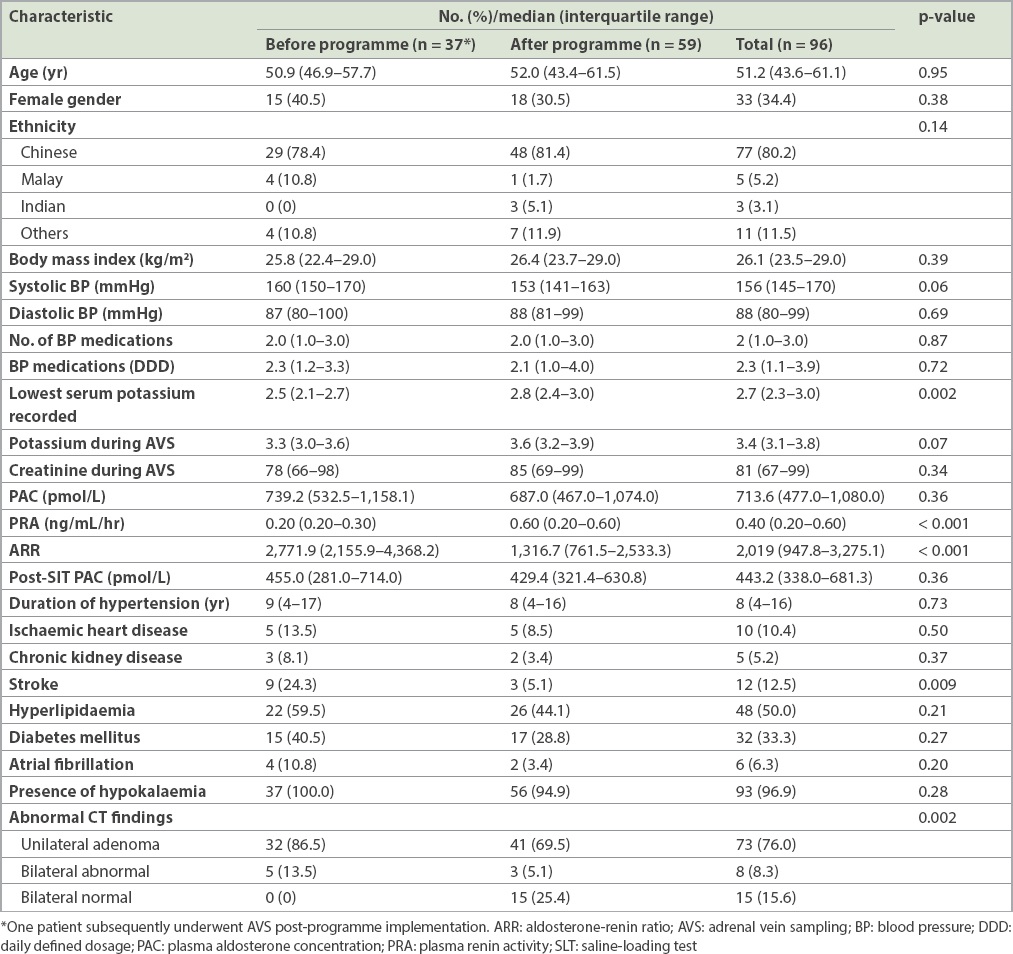
In total, 41 AVS procedures were performed in 37 patients from 1998 to 2014 (2.4 procedures per year), and this increased to 63 procedures in 60 patients from 2015 to 2019 (12.6 procedures per year) (Figs.
Fig. 1
(a) Graph shows the number of AVS procedures performed each year from 1998 to 2019, demonstrating an increase in procedures after programme implementation in 2015 and an increase in successful AVS procedures; from 2015, rapid cortisol reporting was done and five patients with an initial failed AVS underwent successful repeat sampling. (b) Graph shows the number of diagnoses of unilateral or bilateral primary aldosteronism made after AVS procedures. Indeterminate results were mostly due to failed AVS procedures, while two cases in 2019 had successful AVS but bilaterally low aldosterone levels, and subsequently had a conclusive diagnosis on repeat AVS in a separate procedure. AVS: adrenal vein sampling
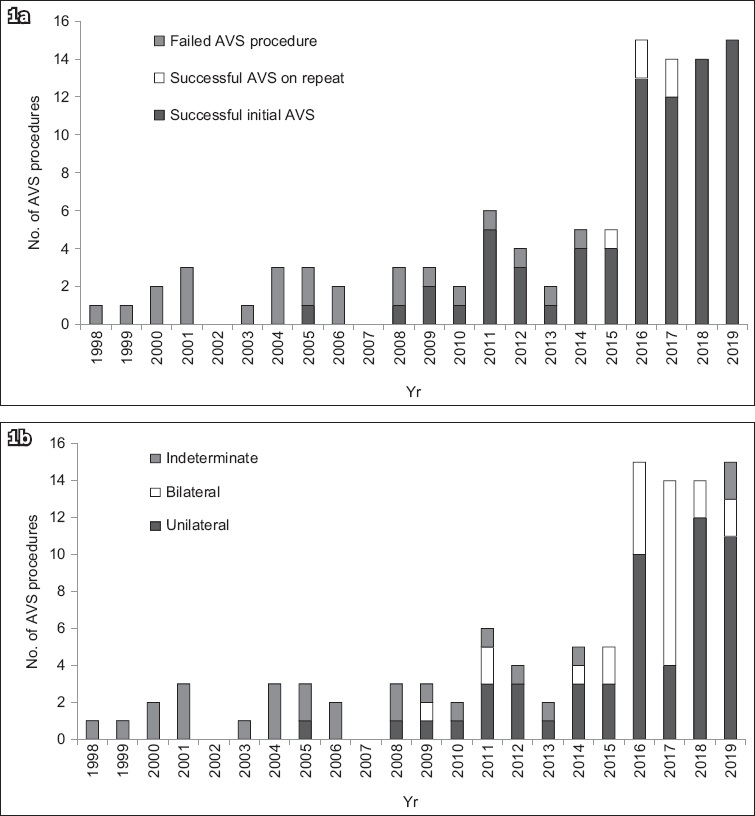
Fig. 2
Flowchart shows the number of patients and AVS procedures performed before and after programme implementation. One patient who had a failed AVS procedure before programme implementation subsequently had a successful procedure after programme implementation. *AVS repeated because results were indeterminate. AVS: adrenal vein sampling; PA: primary aldosteronism
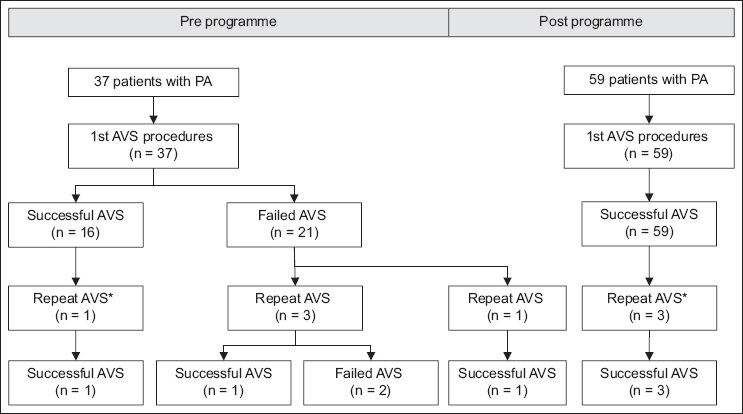
After 2016, two right adrenal vein samples were taken; in 8 (12.7%) cases, the second right adrenal sample was successful, whereas the first sample was not. Hence, this additional sample avoided an additional procedure in 12.7% of cases. Improved AVS success led to more patients (p < 0.01) being identified with unilateral PA, from 12 (32.4%) out of 37 patients to 40 (66.7%) out of 60 patients (
Fig. 3
Pie chart shows that results were concordant (white) and discordant (shaded) between AVS and CT imaging in 76 patients who underwent successful AVS. AVS: adrenal vein sampling; CT: computed tomography; CT bilat Ab: bilaterally abnormal adrenal glands on CT; CT bilat N: bilaterally normal adrenal glands on CT
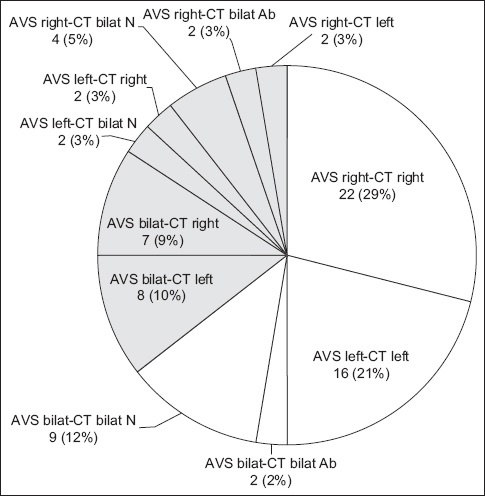
Importantly, there were 4 (5.3%) cases where AVS and CT lateralised to opposite sides. One was a 34-year-old patient whose CT image showed left adrenal gland thickening (Appendix, Supplementary Fig. 1 & Table II). AVS results showed hypersecretion on the right side. Two sequential right adrenal samples were congruous, rendering it unlikely that the right and left samples were swapped. One year after right adrenalectomy, the patient underwent biochemical cure of PA. His blood pressure was 120/83 mmHg on losartan 50 mg daily.
DISCUSSION
We demonstrated that a dedicated effort to implement an AVS programme can improve success rates in a low-volume centre, resulting in more patients being offered curative unilateral adrenalectomy. Although some interventions such as rapid cortisol assays can improve the success of AVS, the response to such interventions has been variable, and they are not possible without certain infrastructure.(6,7) Our success was likely attributable to the multiple interventions made, as opposed to a single intervention. These measures can be adopted in many centres worldwide to achieve similar success.
Rapid cortisol assays can improve the success of AVS by providing immediate feedback of successful cannulation to the operator,(7,17,26) with some assays providing results within six minutes.(16,27) However, this assay is currently not widely available. As an alternative, we engaged our laboratory to provide urgent cortisol reporting. Samples were despatched directly to the laboratory, and designated staff ran the cortisol assays immediately (both neat and diluted at 1:20). For serum samples, special BD Vacutainer® rapid serum tubes can be obtained to allow clotting of blood within five minutes. Alternatively, plasma cortisol can be used. The total turnaround time is often within an hour, which allows the patient to be wheeled back to the angiography suite if needed, with the venous sheath in situ. Notably, while urgent cortisol reporting was useful in our early experience, none of the recent 30 procedures since 2018 required a repeat AVS. We also noted that early AVS failures resulted from lack of absolute values reported for cortisol or aldosterone. Close collaboration with the laboratory ensured that this was made routine subsequently.
We introduced a practice of routinely sending two samples from the right adrenal vein for several reasons. It can be difficult to confirm correct catheter placement with the post-contrast venogram alone. In 12.7% of our cases, the first right adrenal sample was unsuccessful, while the second sample was successful, helping patients avoid a repeat procedure. Our young patient (Appendix, Supplementary Fig. 1 & Table II) had discordant CT and AVS findings. While it is suggested that AVS can be avoided in patients aged < 35 years with an obvious adenoma on CT,(2) the patient’s left adrenal enlargement did not constitute a clear adenoma. The fact that both his samples from the right were congruous for right-sided lateralisation provided added assurance that the right and left samples had not been switched unintentionally. Having a dedicated radiologist to perform AVS allows greater familiarity and increased confidence. CT imaging before AVS has been shown to improve success rates,(28) and can be paramount for identifying variations in both right(29) and left adrenal veins.(30) In our practice, contrast was not routinely given for CT adrenals unless a lipid-poor adenoma was detected on plain CT. Hence, it is important to specifically request for intravenous contrast to delineate the adrenal veins, which are best seen in the portal venous phase.(31) Intraprocedural C-arm cone-beam CT may help but is more time-consuming, increases radiation exposure and may not be widely available.(27)
Factors such as proper catheter equipment and procedural technique should not be understated. As it is more challenging, cannulation of the right adrenal vein should be done first, followed by left and then peripheral cannulation. This reduces time and stress-induced fluctuations between the three samples. The use of different-shaped catheters for the right and left adrenal veins is also important. We routinely created two 1-mm side holes approximately 2 mm from the catheter tips. This can prevent collapse of the vein and catheter displacement owing to the negative pressure created during suctioning while aspirating.(21) We routinely use the 65-cm 4-Fr C2 Cobra catheter (Cordis, Hialeah, FL, USA) to select the right adrenal vein and the 4-Fr Simmons 2 glide catheter (Terumo, Shibuya, Tokyo, Japan) for the left adrenal vein, and the catheters used may vary depending on the patients’ anatomy. Other catheters used include the 5-Fr Cobra, 4-Fr Simmons 1 and 5-Fr Cook Beacon Tip CHG 2.5 (Cook Medical, Bloomington, IN, USA) catheters for the right adrenal vein, and the 5-Fr Cook Beacon Tip CHG 2.5 or Simmons 3 catheter for the left adrenal vein. Microcatheters were not used as they may result in over-selective cannulation of the adrenal veins and increase procedure cost and sampling time. As there is a lack of immediate feedback to the operator regarding catheter positioning and unsuccessful sampling, it is imperative that multimodal imaging be used before and during AVS to allow anatomical confirmation of the adrenal veins and their branches. We suggest using check venograms before and immediately after sampling to evaluate for displacement of the catheter tip.
We acknowledge several limitations to our study. Firstly, the AVS programme implemented included concurrent multiple interventions. As such, it is difficult to elucidate the effect of each intervention on the overall success of our AVS. Nevertheless, we believe that most of these measures can and should be implemented, and would allow other low-volume centres to achieve similarly high success. Secondly, we used an SI > 3, as recommended by expert consensus,(24,32) but some centres recommend an SI > 5. If we had used > 5 as the cut-off, only one procedure after the programme would have been considered unsuccessful, as the left adrenal sample had an SI of 4.7. Thirdly, while we reported our centre as a low-volume centre with 2.4 procedures per year initially, case numbers have subsequently increased. This reflects the increased confidence of physicians with the AVS operators and that success begets success. In line with this, there were no differences in patient baseline characteristics before and after the programme, except for a higher proportion of patients with either bilaterally abnormal or normal adrenals. Hence, to improve AVS success, it is important for clinicians to continually refer patients for AVS and increase the experience of their operators.
Finally, we found a discordance between CT and AVS in 35.5% of our patients (
In conclusion, with adherence to our measures in a standardised AVS programme, we were able to achieve a high success rate of 100% bilateral cannulation in our low-volume centre. Many of our measures are easy to implement and we believe that our achievement can be replicated in many other centres worldwide. This will help identify more patients with curable unilateral primary aldosteronism.
ACKNOWLEDGEMENTS
We would like to thank the Changi General Hospital laboratory department for their kind assistance with rapid cortisol reporting for adrenal vein sampling, Ms Amanda Tan Hong Dan for her assistance in data analysis, and other interventional radiologists at the institute for performing the AVS procedure.


