Abstract
INTRODUCTION
The effects of reduction of left ventricular (LV) systemic afterload following aortic valve replacement (AVR) for severe aortic valve stenosis (AS) were investigated, using echocardiography and tissue Doppler imaging (TDI).
METHODS
We compared the preoperative and postoperative echocardiographic assessments of 23 patients with severe AS who had undergone isolated AVR (n = 13) or concomitant AVR with coronary artery bypass grafting (CABG) (n = 10). Conventional echocardiographic evaluations and TDI at the lateral mitral annulus were performed.
RESULTS
Echocardiography was performed at a median of 120 (interquartile range: 66–141) days after AVR. There was significant reduction in aortic transvalvular mean pressure gradient after AVR. Although LV dimensions, mass and ejection fraction remained unchanged, LV diastolic and systolic functions improved (as observed on TDI). Early diastolic (E’), late diastolic (A’) and systolic (S’) mitral annular velocities increased significantly (p < 0.05). There was significant improvement in TDI-derived parameters among the patients who had isolated AVR, while among the patients who had concomitant AVR with CABG, only S’ had significant improvement (p = 0.028).
CONCLUSION
TDI was able to detect improvements in LV systolic and diastolic function after AVR for severe AS. There was less improvement in the TDI-derived diastolic parameters among patients who underwent concomitant AVR with CABG than among patients who underwent isolated AVR.
INTRODUCTION
Aortic valve stenosis (AS) is the most common valvular disease in developed countries; it is also the third most common cardiovascular pathology after coronary artery disease (CAD) and hypertension.(1) The natural history of AS is defined by a long asymptomatic phase that may persist for several decades. Progressive left ventricular (LV) dysfunction may result from prolonged LV outflow obstruction.(2) Aortic valve replacement (AVR) is the definitive treatment for severe AS in patients who are symptomatic, who have decreased LV ejection fraction, or who are scheduled to undergo concomitant cardiac surgery.(3,4) As AS and CAD may have similar risk factors, and are both known to have increased incidence and disease progression with increasing age, they often coexist in elderly patients. Patients with severe AS and CAD who are undergoing surgery have significantly higher surgical risk as compared to those with isolated severe AS. However, in the last few decades, the number of patients undergoing concurrent AVR with coronary artery bypass grafting (CABG) has doubled.(5)
Tissue Doppler imaging (TDI) is a useful echocardiographic technique that can be used to assess myocardial tissue velocity and detect subclinical LV systolic and diastolic dysfunctions.(6) In the present study, we aimed to investigate the effects of reduction in systemic afterload on the left ventricle after AVR in severe AS. We compared (a) the preoperative and postoperative echocardiographic parameters, including those measured using TDI; and (b) the subsequent improvements in LV function between patients who underwent isolated AVR and those who underwent concomitant AVR and CABG.
METHODS
A total of 23 patients (14 male, nine female) who underwent AVR for severe AS and had paired echocardiographic studies, including TDI, at the National University Heart Centre, Singapore, were included in our study. Among these 23 patients, 13 underwent isolated AVR, while ten underwent concomitant AVR and CABG. Their mean age was 65 ± 8 years. Patients with atrial fibrillation, pacing devices and significant mitral valve regurgitation were excluded.
All 23 patients were assessed comprehensively on transthoracic echocardiography, before and after AVR. The images were stored on a dedicated workstation for offline analysis. The average of three cardiac cycles was taken for each measurement. Continuous wave Doppler ultrasonography was used to measure aortic transvalvular peak velocity. The mean pressure gradient was calculated from the tracing of the velocity curve. Aortic valve area was derived using the continuity equation.(7) LV end-diastolic and end-systolic volumes and LV ejection fraction were measured according to the American Society of Echocardiography guidelines.(8) LV diastolic function was assessed using transmitral early (E) and late (A) filling velocities, and deceleration time was obtained from pulse wave Doppler interrogation at the mitral leaflet tips. Pulse wave TDI was performed in apical four-chamber view to assess longitudinal myocardial velocity.(9) Peak velocities during systole (S’), and early (E’) and late (A’) diastole at the lateral mitral annulus were measured, and E/E’ ratio was calculated. Conventionally, TDI parameters are obtained over both the septal and lateral mitral annulus and the average of the values is used. However, TDI parameters at the septal annulus were excluded in the present study due to the known effect of CABG on these readings.
Data collection and statistical analysis were performed using Microsoft Excel for Windows 2010 (Microsoft, Redmond, WA, USA). Statistical tests of significance were performed and a p-value < 0.05 indicated statistical significance. Continuous variables were presented as mean ± standard deviation or median (interquartile range [IQR]), depending on the normality of the distribution, and analysed using the two-tailed Student’s t-test (for variables with continuous distributions) or Mann-Whitney U test (for variables with unknown distributions). Categorical or dichotomous data was presented as number (percentage) and analysed using chi-square tests.
RESULTS
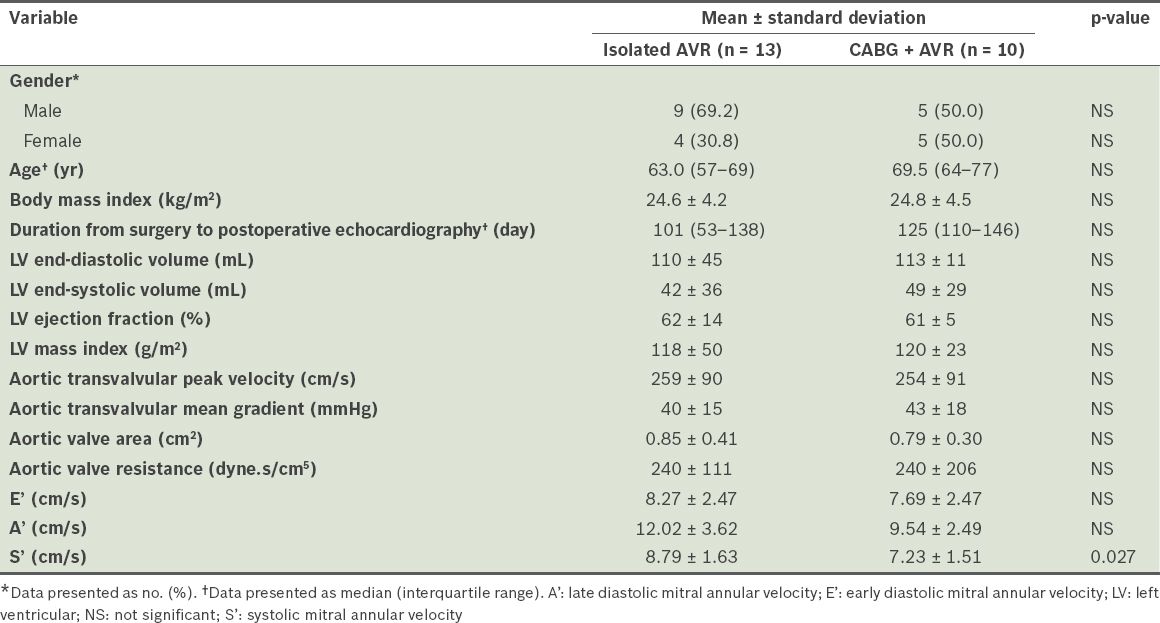
The demographics of the 23 patients, according to whether they underwent isolated AVR (n = 13) or AVR with CABG (n = 10), are listed in
Table I
Demographics of patients who underwent isolated aortic valve replacement (AVR) or AVR with coronary artery bypass grafting (CABG) (n = 23).
After AVR, there was a significant increase in aortic valve area (p < 0.001) and a significant decrease in aortic transvalvular mean pressure gradient (p < 0.001) (
Table II
Comparison of echocardiographic parameters of all patients (n = 23) before and after aortic valve replacement (AVR).
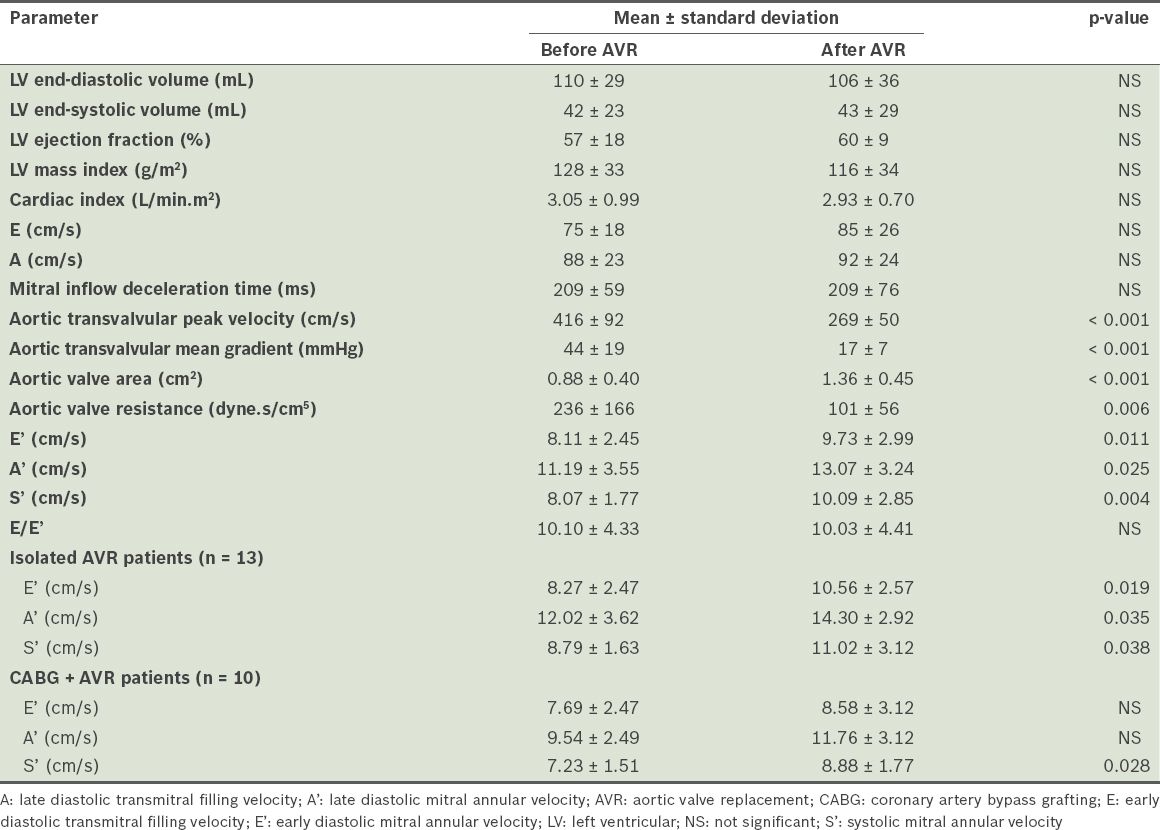
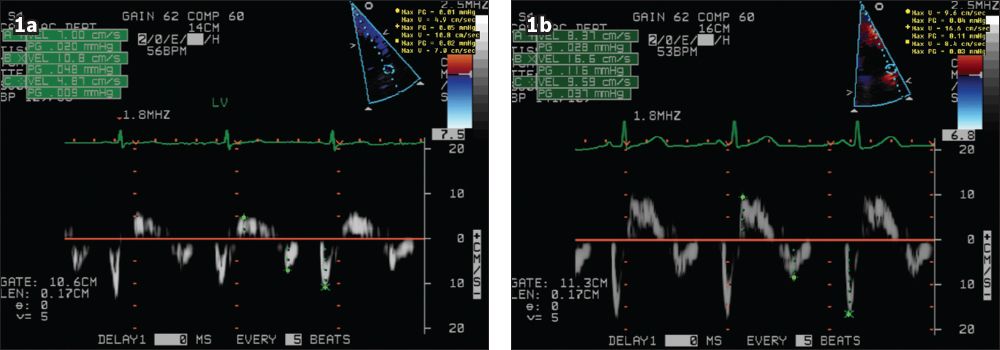
Fig. 1
Tissue Doppler images show the improvement in mitral annular velocities (a) before and (b) after aortic valve replacement.
DISCUSSION
The present study is one of several studies that assessed the improvements in LV diastolic and systolic function among patients with severe AS after AVR. The results (obtained using TDI) of the patients who underwent isolated AVR and those who underwent concomitant AVR with CABG were also compared in the present study. We demonstrated that TDI can be used to detect improvements in LV diastolic and systolic function in patients with severe AS after AVR. TDI has been shown to be useful in detecting subtle alterations in myocardial function(6) and evaluating regional systolic LV contraction.(9-11) The use of TDI may be better than conventional measurements which rely on endocardial movement and wall thickening.(9,10) Furthermore, in conventional echocardiographic assessments, global LV diastolic function can only be indirectly derived from the parameters of LV filling.(11,12) As TDI can provide measurements of myocardial tissue velocity, it allows for the quantitative assessment of both regional systolic and diastolic LV function.(13,14) The motions caused by longitudinally directed fibres that are found mainly in the LV subendocardium and subepicardium can be assessed using myocardial systolic annular velocities obtained from TDI.(15) These motions also contribute to global long-axis function.(16,17) Diastolic annular velocities from TDI can also be used to assess LV diastolic function. In the present study, we showed that there was a significant increase in E’, A’ and S’ after AVR, which is consistent with past literature demonstrating improvement in LV diastolic and systolic function after AVR in patients with severe AS.(18,19)
In severe AS, chronically increased afterload leads to progressive LV remodelling and myocardial hypertrophy.(20) Despite this compensatory mechanism, which reduces LV end-systolic wall stress, increased metabolic demand, impaired LV relaxation and reduced LV compliance may occur in the long run. This results in the inability of the left ventricle to attain normal filling pressure during diastole. The increase in LV diastolic pressure leads to an increased dependence on atrial contribution to LV filling. There is progressive dilatation of the left atrium, resulting in progressive atrial dysfunction.(21,22) A’ is a good predictor of clinical outcomes; a lower A’ suggests reduced atrial booster pump function.(21) Similarly, increased left atrial volume is a reproducible surrogate marker of adverse cardiovascular outcomes.(23) AVR reduces the pressure overload caused by severe AS, thus inducing a trend toward the normalisation of LV haemodynamics, with progressive remodelling of fibrotic and myocyte components.(24)
A previous study that compared patients who underwent isolated AVR and patients who underwent AVR with concomitant CABG showed that the former group had an excellent long-term prognosis, with significant improvement in LV diastolic and systolic function after AVR.(25) This is consistent with the results of the present study; we observed significant improvements in E’, A’ and S’ among the patients who underwent isolated AVR. Among the patients who underwent concomitant AVR with CABG, the only significant improvement was in S’. The baseline TDI parameters of the patients who underwent concomitant AVR with CABG were also lower than those of the patients who underwent isolated AVR. The former group of patients may have more comorbidities, and worsened LV systolic and diastolic functions in the presence of CAD, which confounded the outcomes.(26,27) An alternative explanation would be that more time is needed for recovery of myocardial function in this group of patients. It is possible that since the S’ of this patient group has a significantly lower baseline level (p < 0.01) (as compared to those who underwent isolated AVR), it may have been the first parameter to improve after AVR. However, we did not conduct longitudinal follow-up on the patients and were not able to investigate this.
The present study was not without limitations. The sample size of the study was small. Secondly, the durations between the preoperative and postoperative echocardiographic examinations varied due to the retrospective nature of the study. Nonetheless, there was no significant difference between the patients who underwent isolated AVR and those who underwent AVR with concomitant CABG (
To conclude, TDI is able to detect improvements in LV systolic and diastolic functions in patients with severe AS after AVR. There was less improvement in the TDI-derived diastolic parameters among patients who underwent concomitant AVR with CABG than among those who underwent isolated AVR.


