Abstract
INTRODUCTION
Although ketamine is one of the commonest medications used in procedural sedation of children, to our knowledge, there is currently no published report on predictors of respiratory adverse events during ketamine sedation in Asian children. We aimed to determine the incidence of and factors associated with respiratory adverse events in children undergoing procedural sedation with intramuscular (IM) ketamine in a paediatric emergency department (ED) in Singapore.
METHODS
A retrospective analysis was conducted of all children who underwent procedural sedation with IM ketamine in the paediatric ED between 1 April 2013 and 31 October 2017. Demographics and epidemiological data, including any adverse events and interventions, were extracted electronically from the prospective paediatric sedation database. The site of procedure was determined through reviewing medical records. Descriptive statistics were used for incidence and baseline characteristics. Univariate and multivariate logistic regression analyses were performed to determine significant predictors.
RESULTS
Among 5,476 children, 102 (1.9%) developed respiratory adverse events. None required intubation or cardiopulmonary resuscitation. Only one required bag-valve-mask ventilation. The incidence rate was higher in children aged less than three years, at 3.6% compared to 1.0% in older children (odds ratio [OR] 3.524, 95% confidence interval [CI] 2.354–5.276; p < 0.001). Higher initial ketamine dose (adjusted OR 2.061, 95% CI 1.371–3.100; p = 0.001) and the type of procedure (adjusted OR 0.190 (95% CI 0.038–0.953; p = 0.044) were significant independent predictors.
CONCLUSION
The overall incidence of respiratory adverse events was 1.9%. Age, initial dose of IM ketamine and type of procedure were significant predictors.
INTRODUCTION
Intramuscular (IM) ketamine is widely used in procedural sedation for paediatric patients in the emergency department (ED)(1-6) owing to its efficacy and safety as a dissociative agent for various painful and emotionally disturbing procedures in the ED.(7-10) Unlike other sedative agents, ketamine acts by inducing a ‘trance-like cataleptic state’, leading to sedation, analgesia and amnesia,(11) while allowing patients to retain protective airway reflexes, spontaneous respiration and cardiopulmonary stability. Owing to this unique mechanism of action, ketamine has been shown to have the lowest incidence of serious adverse events among sedative agents.(12) Its superior safety profile has made it an especially valuable sedative agent for the paediatric population, who undergo procedural sedation more frequently in the ED and generally have higher risks of sedation-related adverse events compared to adults.(13)
Despite its safety and efficacy, ketamine sedation still carries certain risks. These include respiratory adverse events such as oxygen desaturation, apnoea and laryngospasm as well as emesis, recovery agitation and clonus.(9,14-16) Although ketamine-related respiratory adverse events are seldom encountered, they can lead to devastating outcomes if not managed well.(13) An individual-patient data meta-analysis of 8,282 children by Green et al(7) showed an overall incidence of respiratory adverse events at 3.9% and identified age and co-administration of benzodiazepines as significant predictors. However, both IM and intravenous (IV) ketamine were included in that study and, to our knowledge, no such study has been conducted solely on Asian children using IM ketamine alone. Hence, the present study aimed to determine the incidence of and factors associated with respiratory adverse events in children undergoing procedural sedation with IM ketamine in a paediatric ED in Singapore.
METHODS
A prospective electronic database of all paediatric sedations performed in the ED of KK Women’s and Children’s Hospital, Singapore, was set up in April 2013. The database contains information from the electronic medical records and sedation adverse event charting forms, which is prospectively entered. For all patients undergoing procedural sedation in the ED, the adverse event charting form is used to record all intraprocedural adverse events and subsequent interventions. It includes a checklist of the types of adverse events (i.e. respiratory, gastrointestinal, cardiovascular, neurological, others or death).
Types of respiratory adverse events included: oxygen desaturation, central apnoea, partial obstructive apnoea, complete obstructive apnoea, laryngospasm and clinically apparent pulmonary aspiration syndrome. Interventions performed included: vigorous tactile stimulation, airway repositioning, suctioning, supplemental oxygen, bag-valve-mask ventilation, oral airway, cardiopulmonary resuscitation (CPR) and intubation. Patients were not routinely given supplemental oxygen during the procedure.
A retrospective study was conducted on all patients who underwent procedural sedation with IM ketamine as the primary sedative agent from 1 April 2013 to 31 October 2017. Patients who received IV ketamine or another primary sedative agent such as nitrous oxide were excluded from the study. The study protocol was approved by the hospital’s institutional review board.
For each patient, information extracted from the electronic database included patient demographics, time of arrival in the ED, diagnosis, procedure, medications given, intraprocedural adverse events (if any), interventions (if any) and subsequent disposition. The site of procedure (e.g. upper limb, face, lower limb) was determined from the patient’s electronic medical records. The primary outcomes measured were the types of respiratory adverse events and the interventions required to manage the adverse events. Oxygen desaturation was defined as oxygen saturation (SpO2) less than 94%.
Prior to statistical analysis, the Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) data file was anonymised by removing all patient identifiers. A unique case ID was assigned to each patient on the anonymised Excel data sheet. Further categorisation and coding were performed on the anonymised database for the following variables: time of arrival in the ED (0800–1600 hours, 1600–0000 hours or 0000–0800 hours), diagnoses, procedures, initial dose of IM ketamine (3 mg/kg or 4 mg/kg), and any top-up or additional doses. All other medications administered were categorised into local anaesthesia (LA), additional sedative or additional analgesia. Additionally, the use of parenteral morphine prior to sedation was coded for separate analysis.
Statistical analysis was performed using IBM SPSS Statistics version 19.0 (IBM Corp, Armonk, NY, USA). Descriptive statistics were used for the incidence of respiratory adverse events and the baseline characteristics of patients. All categorical variables were analysed using chi-square test, while independent t-test was used for continuous variables. Subsequently, univariate and multivariate regression analyses were performed, with respiratory adverse event as the primary outcome. Univariate analysis was conducted for the following variables: age, gender, race, time of arrival in the ED, dose of IM ketamine, top-up/additional dose of IM ketamine, failed LA, additional analgesia, morphine, diagnosis, procedure and the site of procedure. Variables with p-value < 0.1 in the univariate analysis were included in a multivariate logistic regression model to identify the independent predictors. Statistical significance for the multivariate analysis was set at p < 0.05. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported.
RESULTS
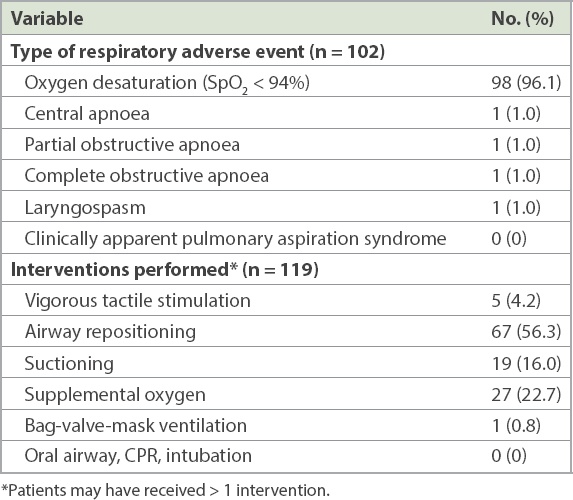
A total of 5,476 patients who underwent procedural sedation with IM ketamine as their primary sedative agent were enrolled in the study. 102 (1.9%) children experienced respiratory adverse events (
Table I
Respiratory adverse events and interventions.
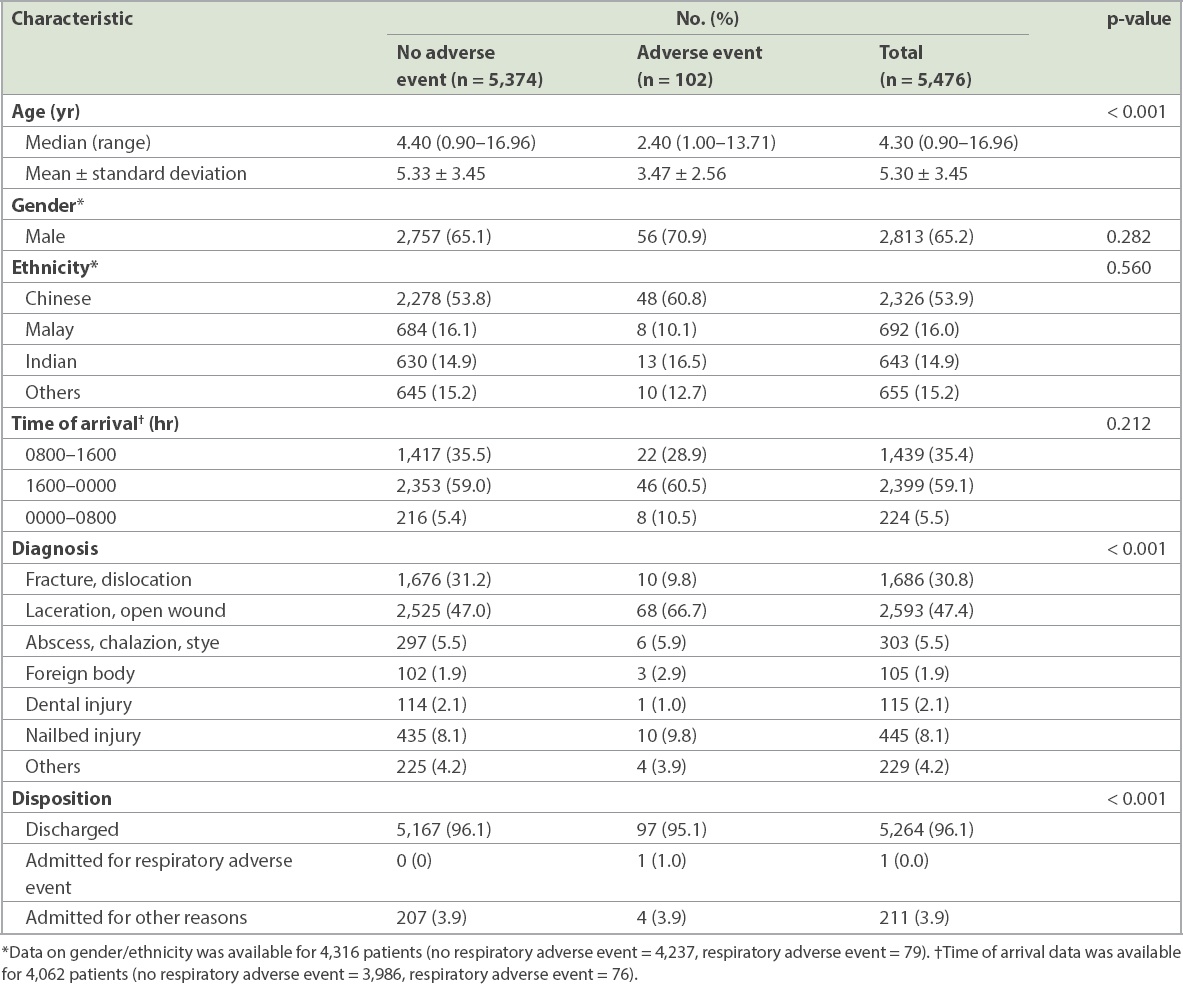
The baseline characteristics of patients with and without respiratory adverse events are presented in
Table II
Baseline characteristics of patients with and without respiratory adverse events.
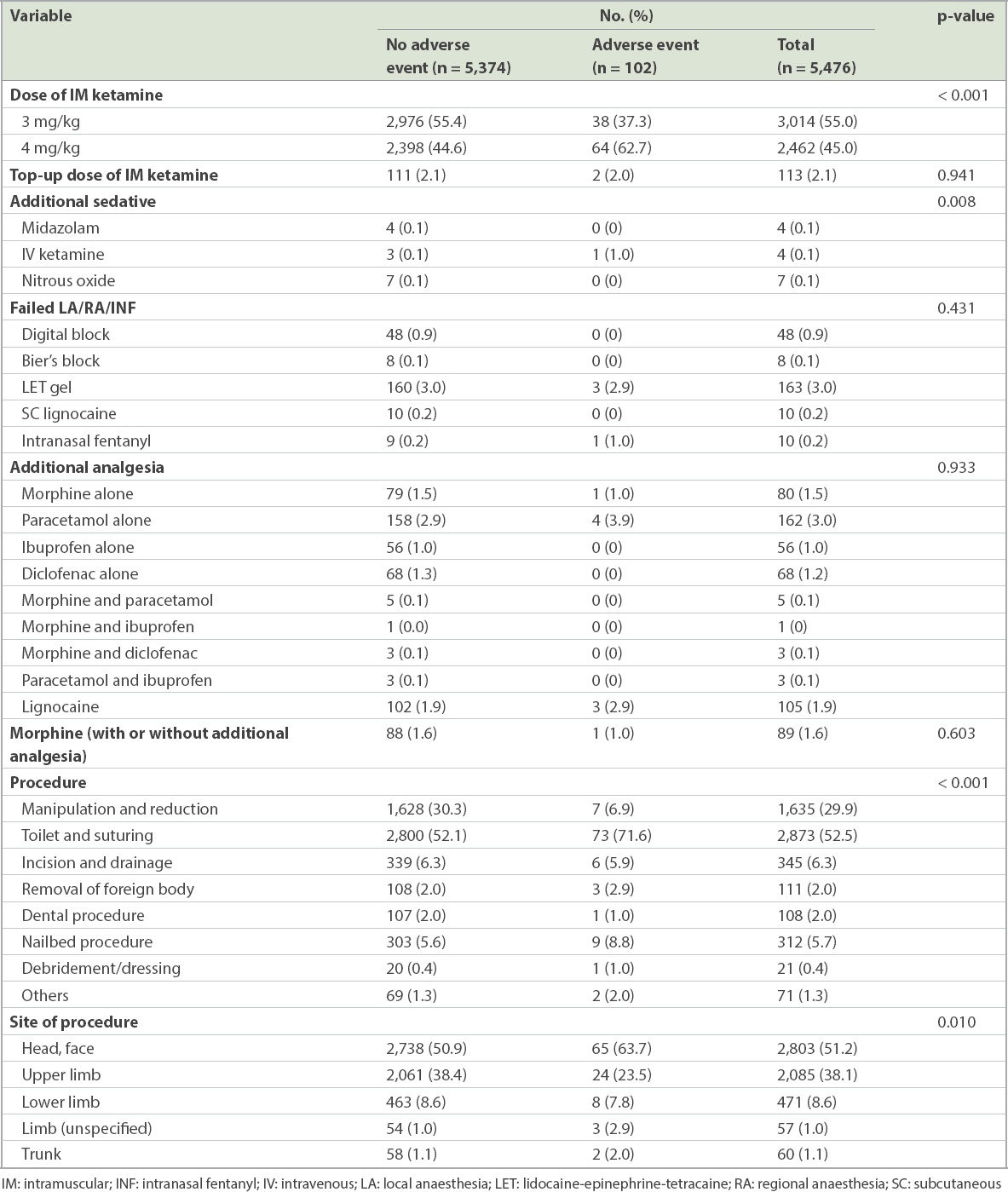
The frequency distribution of medications administered and procedures performed for the two outcome groups are shown in
Table III
Medications and procedures for patients with and without respiratory adverse events.
Results of the univariate and multivariate logistic regression analyses are shown in
Table IV
Predictors of respiratory adverse event.
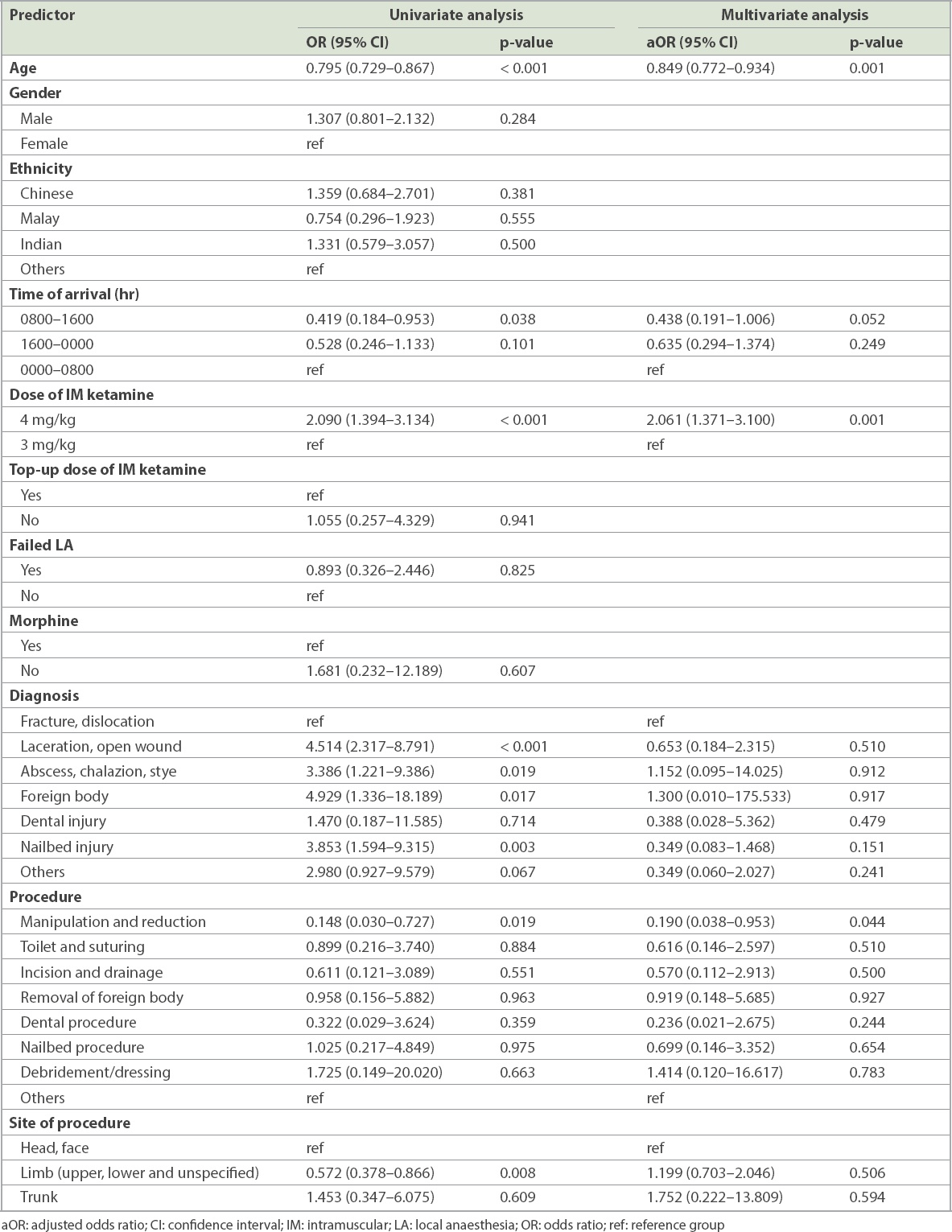
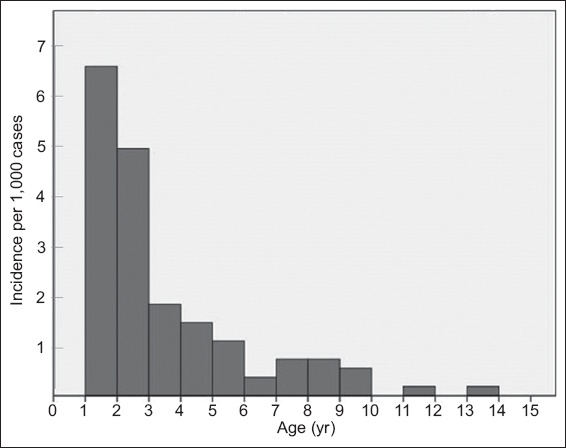
Fig. 1
Bar chart shows distribution of respiratory adverse events stratified by age.
DISCUSSION
To our knowledge, this is the largest single-centre study of respiratory adverse events in procedural sedation using IM ketamine in an Asian country, with a recruitment of 5,476 patients over a five-year period. The nature of a single-centre study eliminates possible discrepancies in methodology, which is a limitation of multinational studies, and allows for greater generalisability to our local paediatric population.
Overall, we have demonstrated that IM ketamine is a safe drug for procedural sedation in the paediatric population. Consistent with the existing literature,(7,17-19) we found a low incidence of respiratory adverse events of 1.9%. Multiple factors such as differences in study population, definitions of respiratory adverse events and inclusion of IV ketamine in other studies could possibly account for the small discrepancies in incidence rates.
Oxygen desaturation accounted for the majority of these respiratory adverse events. Oxygen desaturation is considered a minor respiratory adverse event when SpO2 is between 75% and 90%(20) owing to its transient nature and easy reversibility using simple interventions such as airway repositioning, suctioning and supplemental oxygen. The threshold for oxygen desaturation based on non-invasive SpO2 measurement varies among institutions. The World Health Organization guidelines for oxygen therapy in children recommend starting oxygen therapy when SpO2 drops below 90%, to prevent tissue hypoxia, and when it drops below 94% if there are co-existing conditions that affect oxygen delivery, such as severe anaemia.(21) We chose an SpO2 threshold of < 94% for this study, as interventions were consistently performed at this level. A separate analysis using an SpO2 threshold of ≤ 95% yielded a slightly higher incidence of 2.2% and had similar predictors of younger age (< 3 years) and higher dose of IM ketamine (4 mg/kg vs. 3 mg/kg). As the incidence of respiratory adverse events is low (1.9%), we do not suggest routine oxygen supplementation for procedural sedation. Routine oxygen supplementation without end-tidal carbon dioxide (ETCO2) monitoring may also delay the detection of any respiratory adverse events.
Serious respiratory events are again found to be uncommon. We observed a lower incidence of serious respiratory events compared to a large observational cohort review of 22,645 children by Grunwell et al.(17) In their study, the frequency of serious adverse events, including airway obstruction, laryngospasm, emergent airway intervention, aspiration and cardiac arrest, was 1.77%. Bhatt et al,(12) in their multicentre, observational cohort study conducted in six paediatric EDs in Canada, reported that the use of ketamine hydrochloride alone resulted in the lowest incidence of serious adverse events (0.4%) and significant interventions (0.9%). A meta-analysis and systematic review published in 2016(22) reported an incidence of 4.2 per 1,000 sedations for the adverse event of laryngospasm when ketamine was administered alone, and an even higher incidence (4.6 per 1,000) when it was combined with propofol. By contrast, our study had a lower incidence of serious respiratory adverse events of 0.07%, or 0.3% when the ten cases of desaturation below 75% were included. The laryngospasm risk in our cohort was 0.02%. The strict sedation criteria of age one year and above and no active upper respiratory tract infection may have accounted for the lower incidence rate of respiratory adverse events. Lastly, no patient required significant intervention (CPR or intubation), including dental patients. As such, we advocate for the continuation of IM ketamine as one of the first-line sedatives for paediatric sedation in the ED, using an initial dose of 3 mg/kg or 4 mg/kg.
Age was found to be a significant positive predictor of respiratory adverse events in children. This concurs with previous studies that found age to be a significant predictor(7,17) and can be explained by the innate anatomical and physiological airway differences at varying ages. Younger children have a larger occiput, relatively large tongue, enlarged adenoids and higher metabolic demands, resulting in higher risks of hypoxia following apnoea, making them more vulnerable to respiratory adverse events. In contrast to existing studies, we identified an older age cut-off of three years: children below this age were found to be about 3.6 times more likely to experience a respiratory adverse event following ketamine sedation compared to children aged three years and older. Clinically, it would be important to identify these patients and anticipate a higher risk of respiratory adverse events when performing ketamine sedation for children in this vulnerable age group.
A higher initial dose of IM ketamine of 4 mg/kg, as opposed to 3 mg/kg, was the second significant positive predictor of respiratory adverse events in our cohort. We found that patients who were administered a dose of 4 mg/kg were twice as likely to experience respiratory adverse events during ketamine sedation compared to those who were administered a 3-mg/kg dose. This was unexpected, as ketamine is known to not follow a dose-response continuum, unlike other sedative agents, and hence, the quantity of ketamine given should not lead to different respiratory outcomes.(1) Similarly, the meta-analysis by Green et al did not find any proportional dose-related increase in risk.(7) Interestingly, the same study reported that a sub-dissociative IM dose of less than 3 mg/kg was associated with significantly fewer respiratory adverse events. A study comparing two sub-dissociative doses of 2.0 mg/kg or 2.5 mg/kg by McGlone et al(23) also found a lower rate of airway complications in the lower dose.
Previous studies have identified the co-administration of certain drugs such as anti-cholinergics(7) and benzodiazepines(17) to be associated with respiratory adverse events. In our study, we found that additional sedatives or midazolam, intravenous ketamine or nitrous oxide prior to IM ketamine sedation were not significant predictors. This could be attributed to the very few cases of additional sedatives in our study. The co-administration of morphine was also not associated with respiratory adverse events, although opioids are known to be potent respiratory depressants.
We analysed two procedural factors, namely the type and site of procedure, and their associations with respiratory adverse events. While the site of procedure was not found to be a significant predictor, the type of procedure was significant, in that children who had undergone manipulation and reduction were less likely to have respiratory adverse events. In a previous study, patients undergoing painful procedures had a significantly lower risk of serious adverse events than those not undergoing a painful procedure, which was postulated to be due to the protective effect of a painful stimulus.(17) Thus, the increased pain caused by manipulation and reduction procedures, compared to suturing, could be the reason for the lower risk. Oropharyngeal procedures involving airway manipulation, such as upper endoscopy procedures, have also been found to be associated with an increased incidence of laryngospasm,(1,2) while less stimulating dental procedures have not shown an increased risk.(17,24,25) In our ED, the department policy is not to sedate patients undergoing procedures (e.g. gastroendoscopy) that stimulate the posterior pharynx with ketamine, owing to higher risk of laryngospasm.
Based on the findings of our study, we suggest that practitioners ensure that more precautions are taken in sedating young children below the age of three years and that parents are counselled about the increased risk of respiratory adverse events in this age group. Although a lower (3 mg/kg) dose of IM ketamine can be considered in this more vulnerable age group, we recommend that this should not be at the expense of inadequate sedation, especially for procedures that require a more prolonged period of sedation.
Our study was not without limitations. Firstly, as it was an observational study, direct causal conclusions should not be drawn from our results. Moreover, as this study was conducted in the ED of a paediatric tertiary centre, the results cannot be generalised to an ambulatory clinic setting. We did not further analyse the data to study the effects of emesis on oxygen desaturation, although sedation with ketamine alone is associated with higher rates of emesis compared with other sedatives.(3) Lastly, as all the patients undergoing procedural sedation with ketamine in the department fasted for three hours post intake of solids, we were unable to study the effect of fasting time or lack of fasting against the risk of respiratory adverse events.
In conclusion, we found that the overall incidence of respiratory adverse events during IM ketamine sedation in paediatric patients in an ED was 1.9%. Age below three years and a higher initial dose of IM ketamine (4 mg/kg) were significantly associated with an increased incidence of respiratory adverse events, while manipulation and reduction procedures were associated with lower risk. As IM ketamine continues to be a common sedative agent in paediatric EDs, we believe that our findings can provide quantitative risk estimates to aid in the selection of patients, risk communication and taking of informed consent during procedural sedation, leading to safer outcomes.


