Abstract
INTRODUCTION
Data on malignancy after kidney transplantation (KTX) is limited in our region, leading to challenges in the care of renal allograft recipients. We aimed to examine the epidemiology, risk factors and outcomes of post-KTX patients.
METHODS
A retrospective cohort study was conducted of 491 patients who underwent KTX from 1 January 2000 to 31 December 2011. Data linkage analysis was done between our centre and the National Registry of Diseases Office to determine the standardised incidence ratio (SIR), standardised mortality ratio (SMR) and risk factors for malignancy after KTX.
RESULTS
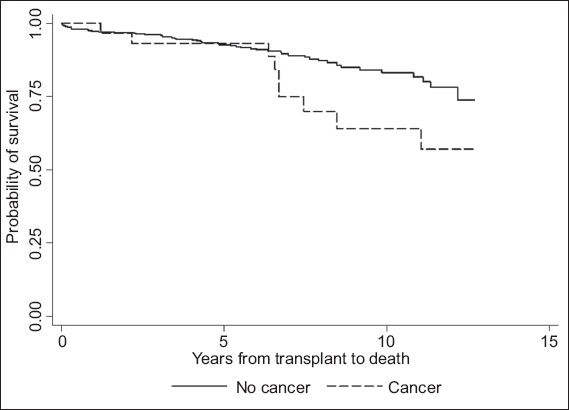
31 patients (61.3% male) developed malignancy during this period, and their median age at diagnosis was 50 (range 18–65) years. Median time to malignancy diagnosis was 2.6 (range 0.3–7.9) years, with cumulative incidence of 1%, 4% and 10% at one, five and ten years, respectively. The commonest malignancy type was lymphoma, followed by kidney cancer, colorectal cancer and malignancy of the male genital organs. Multivariate analysis identified cyclosporine use as an independent risk factor for malignancy. Compared to the general population, KTX recipients had higher malignancy and mortality rates after malignancy diagnosis (SIR 3.36; SMR 9.45). Survival rates for KTX recipients with malignancy versus those without malignancy were 100%, 93% and 64% versus 97%, 93% and 83% at one, five and ten years, respectively.
CONCLUSION
KTX was associated with higher mortality and incidence of malignancy. Newer immunosuppressive agents and induction therapies were not found to be risk factors for malignancy, possibly due to our relatively small sample size.
INTRODUCTION
Despite improvements in patient and allograft survival rates after kidney transplantation (KTX), post-transplant malignancy remains a major adverse outcome and a challenge in the care of renal allograft recipients.(1,2) The elevated risk of malignancies is well recognised in end-stage renal disease patients and post-transplant patients, and such malignancies have a distinct pathogenesis and a complex relationship with immunosuppressant use and viral infection.(3,4) More recently, pre-transplant dialysis and its duration have also been shown to be a significant risk factor for post-transplant malignancy.(5) This is of particular importance in Singapore, where the waiting time for transplantation is reported to be approximately nine years.(6) As such, knowledge of the types of malignancies, as well as the magnitude of increased risk is clinically relevant to KTX recipients.
In Asian countries, different patterns of epidemiology have been reported. Studies from Taiwan and Hong Kong found that urogenital cancers, liver cancers and lymphomas are the commonest types of cancer encountered,(7,8) in contrast to studies from Western countries, where skin cancers, lymphomas and anogenital cancers are more prevalent.(2,9) A lower incidence of skin cancer is characteristic of Asian KTX recipients, which may be attributed to genetic variations in the development of skin cancer.(6,10) A previous study from our centre, Singapore General Hospital (SGH), Singapore, reported that skin, ororespiratory and urogenital malignancies were the commonest malignancies seen in 950 KTX recipients who were studied from 1972 to 1997.(11) Mok et al, in a more recent study evaluating the ten-year outcomes of KTX recipients in our centre, showed that malignancy remained the third commonest cause of death after transplantation.(12)
With the introduction of more potent immunosuppression drugs and progress in transplantation and treatment at our centre, we aimed to determine whether the spectrum of malignancies has changed in the current era of immunosuppression. We also sought to identify the risk factors for post-KTX malignancy and determine the survival rate in this patient group compared to the general population, with a view to facilitating counselling and improving cancer surveillance strategies in KTX.
METHODS
The following study protocol was approved by the SingHealth Centralised Institutional Review Board. We retrospectively reviewed the medical records of all Singapore citizens and permanent residents who underwent KTX in SGH from 1 January 2000 to 31 December 2011. Clinical data was obtained from the medical case notes and electronic medical records of the hospital. Demographic characteristics and information such as the presence of medical comorbidities, detailed transplant and cancer history, immunosuppression regimens, and renal allograft function were collected. The clinical data from SGH was then cross-referenced with data from the Singapore Cancer Registry, National Registry of Diseases Office, to track new malignancies that occurred after transplant. The registry was established in 1968 to collect information on all cancers diagnosed in Singapore. Transplant patients were followed up until the first occurrence of cancer, death or the end of the study period (i.e. 31 December 2012). Cancer death is defined as death primarily due to cancer.
Statistical analysis was performed using Stata version 10.2 (StataCorp, College Station, TX, USA). Apart from describing the sociodemographic and clinical profiles of KTX recipients, we also determined the standardised incidence ratio (SIR) and standardised mortality ratio (SMR) of malignancy after the transplant. SIR was calculated by dividing the number of observed cases of cancer among KTX recipients by the expected number of cancer cases computed using population cancer incidence rates according to age, gender and calendar-year. SMR was derived by dividing the observed rates of death by the corresponding expected rates in the general population. For statistical analysis, the one-, five- and ten-year cumulative incidence of developing cancer and survival rates were analysed using the Kaplan-Meier method. Differences between survival curves were tested by the log-rank test. Univariate and multivariate logistic regressions, adjusted for age, gender and ethnicity, were used to assess the association between risk factors and the development of post-KTX malignancy, with a corresponding 95% confidence interval (CI). For all analyses, a p-value < 0.05 was taken to indicate statistical significance.
RESULTS
A total of 491 KTX recipients were followed up at our hospital between 1 January 2000 and 31 December 2011. Two patients who had a previous history of cancer were excluded from the analyses, leaving 489 eligible patients. The demographic and clinical characteristics of KTX patients with post-transplant malignancy and those without malignancy are listed in
Table I
Baseline demographic and clinical characteristics of the study population (n = 489).
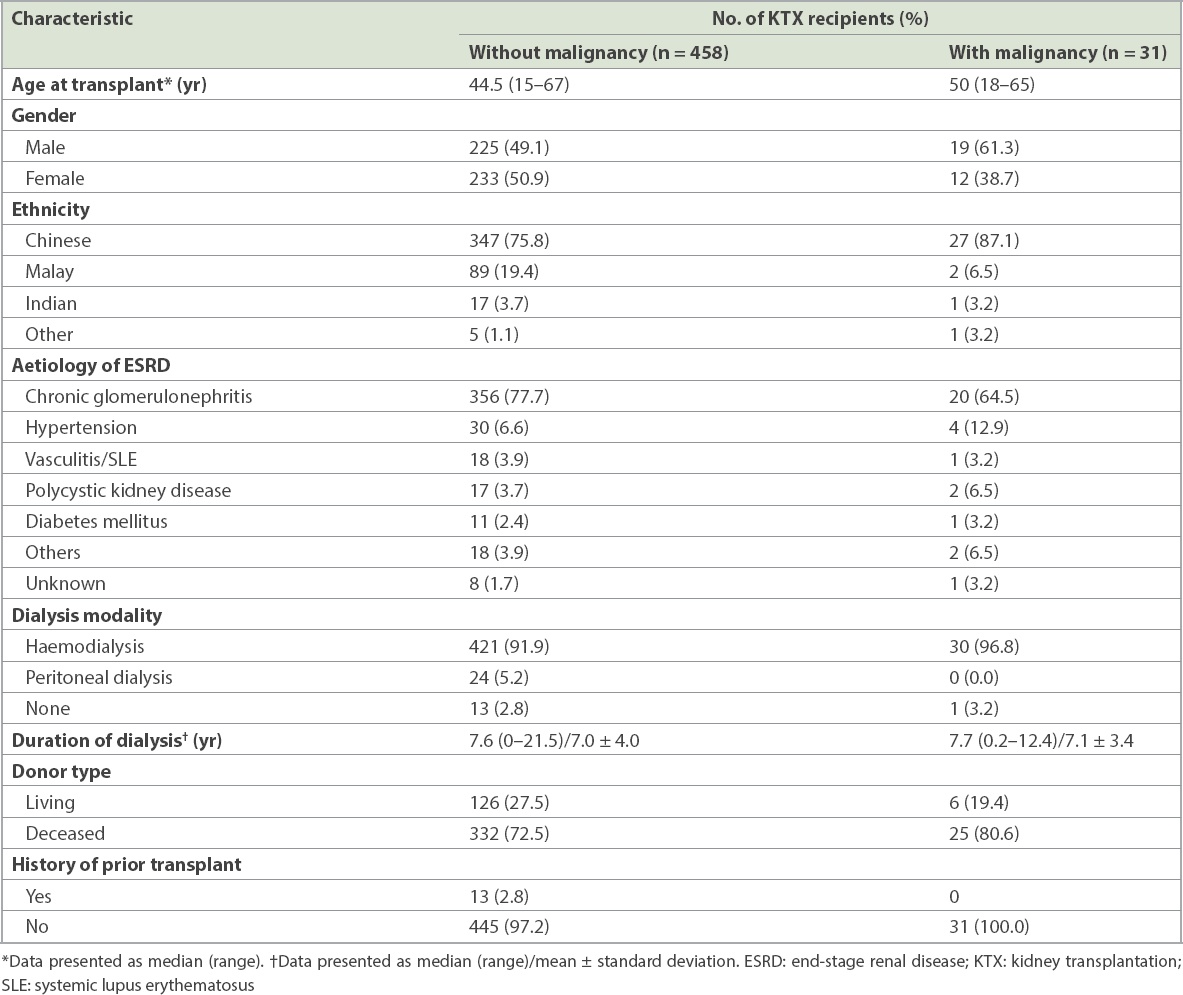
The commonest type of malignancy (
Fig. 1
Graph shows the spectrum of malignancies after kidney transplantation.
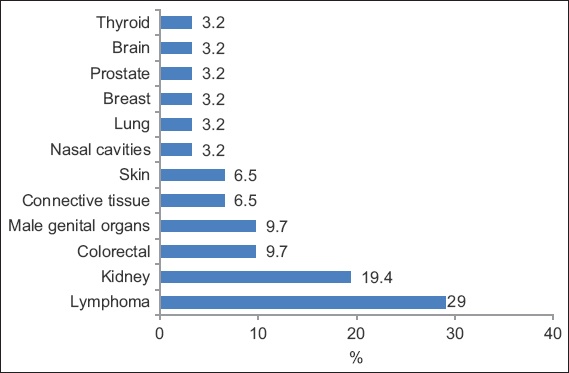
Cox proportion hazard regression analyses were performed to determine the predictors that were associated with post-transplant malignancy (
Compared with the general population, KTX patients had a threefold higher risk of developing post-transplant malignancy (SIR 3.36). The risk was higher in male patients (5.05 vs. 2.32;
Table II
Risk factors of post-kidney transplantation malignancy on univariate and multivariate analyses.
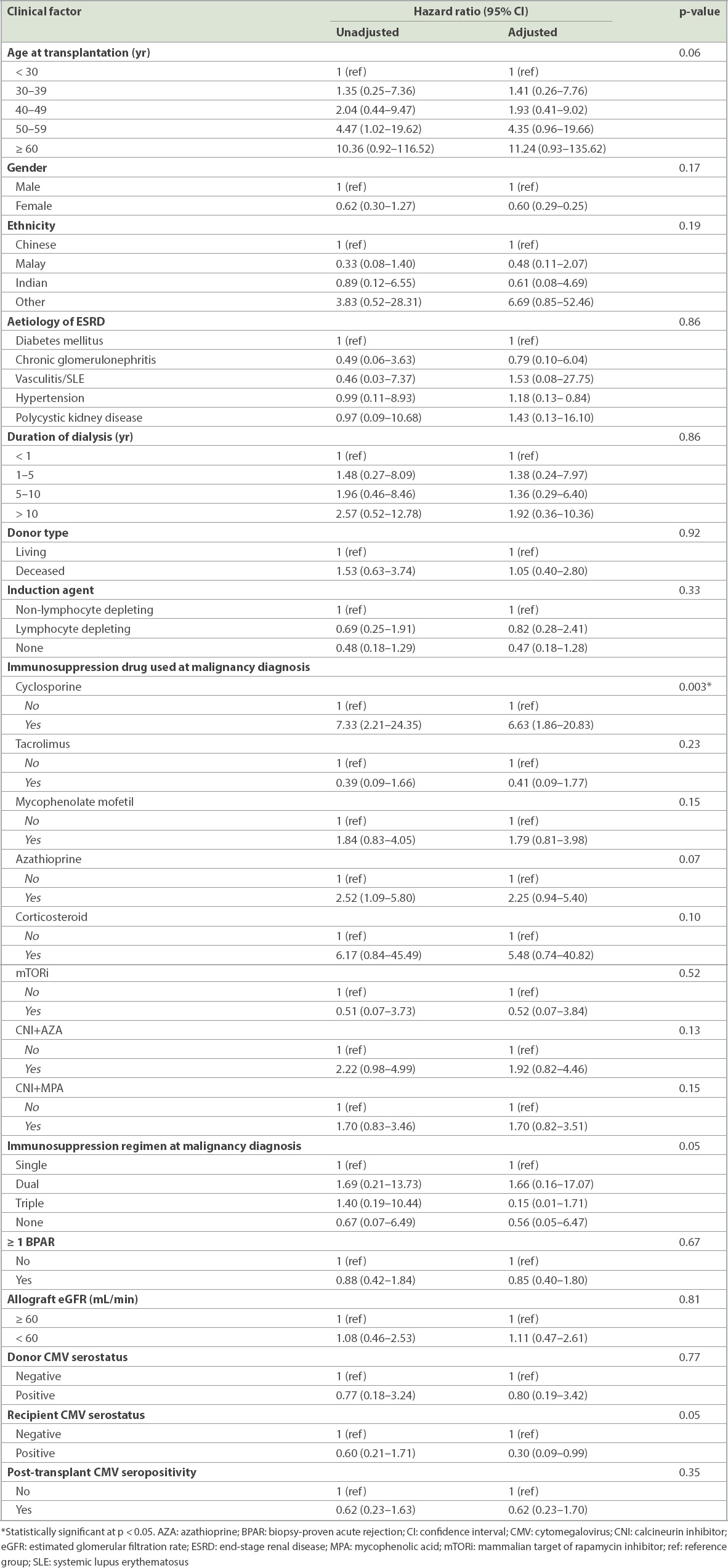
Table III
Standardised incidence ratio (SIR) and standardised mortality ratio (SMR) of kidney transplantation recipients.
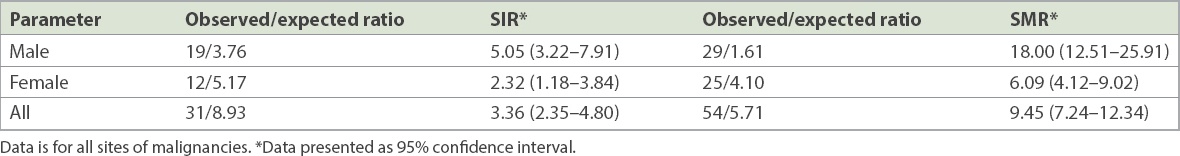
Fig. 2
Kaplan-Meier curve shows the mortality rates of kidney transplantation patients with and without cancer at one, five and ten years.
DISCUSSION
The association between malignancy and KTX has long been known and remains one of the major causes of mortality and morbidity in kidney allograft recipients.(2,13,14) Our local registry data showed that post-transplant malignancy is among the top three leading causes of death.(15) This is similar to the findings in the registry cohort in Australia and New Zealand.(16) Our centre previously reported 23 (2.42%) cases of post-KTX malignancies out of 950 KTX recipients between 1972 and 1997.(11) In our study, KTX recipients were found to have a threefold higher risk of developing post-transplant malignancies (SIR 3.36, 95% CI 2.35–4.80) compared to the general Singaporean population. This is consistent with other Asian populations such as Taiwan (SIR 3.75) and Korea (SIR 3.3)(7,17) but higher than the cancer risk among end-stage renal disease patients on dialysis in Singapore,(18) which may suggest that KTX recipients have unique risk factors.
The most common post-KTX malignancy in our population was PTLD, followed by kidney cancer, colorectal cancer and malignancies of the male genital organs. Similarly, PTLD was found to be the most common cancer among KTX recipients in the Hong Kong Renal Registry.(8) The incidence of lymphoma follows a bimodal distribution after transplantation. In the Australia and New Zealand Dialysis and Transplant Registry, early (defined as < 2 years after the first transplantation) non-Hodgkin lymphoma (NHL) was found to be associated with Epstein-Barr virus (EBV) seropositivity at transplantation and the use of T-cell depleting agent. Late (≥ 2 years) NHL was associated with the use of calcineurin inhibitors, increasing age since transplantation and older age.(19) Lymphoma risk was found to be the highest in the first year and plateaued 4–5 years after transplantation.(20) Unfortunately, we were unable to analyse the association between EBV and PTLD in our study, as the EBV virology data of our recipients was mostly unavailable.
Kidney cancer risk is elevated and bimodal in onset time, as demonstrated in many studies;(20) however, this was not observed in our cohort, whose median age at diagnosis was 48 (range 45–54) years. In Singapore, Goh and Vathsala examined the incidence of RCC in 1,036 KTX recipients and demonstrated that native renal cysts and increased dialysis duration were significant risk factors for RCC; the authors recommended regular ultrasonography (US) surveillance for early detection of RCC.(21) In our study, RCC was found to be the second commonest cancer. Although we screened for RCC in patients with annual US, there is still a lack of evidence that US surveillance improves mortality. The increased incidence of RCC in our population could be related to the longer duration of pre-transplant dialysis as compared to the non-transplant population.(21)
Colorectal cancer is the commonest cancer in the general Singaporean population, accounting for 17.8% and 14.1% of all cancers and annual age-standardised mortality rates of 12.1 and 9.0 per 100,000 in men and women, respectively.(22) In our study, colorectal cancer and malignancies of the male genital organs were the third commonest cancers. In Singapore, the current screening strategy for colorectal cancer begins at age 50 years with tools such as faecal occult blood test or colonoscopy.
Lip cancer, non-melanoma skin cancer and NHL were the commonest post-transplant malignancies reported in the United Kingdom (UK) Registry Audit.(4) In contrast, the incidence of skin cancer was only 6.45% in our cohort, which predominately comprised patients of Chinese ethnicity. Studies have found that a low incidence of skin cancer is characteristic of Asian KTX recipients,(7,8,23) and this appeared to be true of our study as well.
The influence of immunosuppression regimens on cancer has long been a subject of debate and remains one of the major challenges faced by transplant physicians in the long run. In our study, multivariate analysis revealed that cyclosporine use was an independent risk factor in predicting an increased risk of cancer after KTX. Cyclosporine was shown to promote carcinogenesis by interfering with DNA damage through upregulating expressions of tumour growth factor-beta and vascular endothelial growth factor.(24,25) There are conflicting reports in the literature that cyclosporine use increases the risk of post-transplant malignancy. For instance, Shuttleworth et al found that the prevalence rates of cutaneous dysplasia were 22% and 9% in their cyclosporine and azathioprine groups, respectively.(26) However, an analysis of 598 KTX recipients by Vogt et al showed no evident risk of de novo malignancies attributed to cyclosporine.(27) A study by Caillard et al, which specifically examined the risk of immunosuppressive drugs on the development of lymphoproliferative disorders, reported that cyclosporine use was associated with an elevated risk of developing graft lymphoma (relative risk 2.7).(28) More recently, a 2015 study on the clinical impact of cytochrome p450 in KTX found that the CYP3A4*22 allele was significantly associated with the development of cancer after KTX.(29) The relationship of genetic polymorphism in our multiethnic population with CYP3A4*22 and cancer development is still uncertain.
Induction agents are the mainstay of rejection prevention after transplantation. However, studies have suggested a relationship between induction agents and cancer development. Opelz and Henderson examined the incidence of NHL in a multicentre study of 45,141 KTX recipients and found that the risk of developing NHL was increased in patients who received antilymphocyte antibodies for induction or as rejection treatment.(30) This finding is similar to that of Caillard et al, who demonstrated a 1.4-fold increase in the risk of developing PTLD with polyclonal induction.(28) However, our study did not show an increased malignancy risk in lymphocyte-depleting patients. This was consistent with a meta-analysis conducted by Webster et al(31) on 38 trials involving 4,893 KTX recipients, which showed that the risk of malignancy at one year did not pose a significant difference compared to mono- or polyclonal antibodies. However, the authors acknowledged that their study may not have had an adequate time period to detect the emergence of new malignancies.(31) As for maintenance therapy, Bustami et al reported that among those who did not receive an induction agent, a lower incidence of de novo tumours was observed in recipients who received tacrolimus compared to those who received cyclosporine.(32) From our observation, patients on tacrolimus appeared to have a lower incidence of post-transplant malignancy compared to those on cyclosporine, but the difference was not statistically significant due to our small sample size. We were also unable to find any statistically significant difference between patients on azathioprine and those on mycophenolate; this is similar to the finding of Bustami et al.(32) In a study by Gallagher et al, in which 489 recipients were allocated to one of three immunosuppressive regimens (azathioprine and prednisolone; cyclosporine monotherapy; or cyclosporine monotherapy, followed by a switch to azathioprine and prednisolone after three months),(33) no association was found between treatment allocation and the development of non-skin cancer or skin cancer. In our study, we found no statistical difference in incidence of post-transplant malignancy between a combination of calcineurin inhibitor and azathioprine versus calcineurin inhibitor and mycophenolate.
In addition to the effects of long-term immunosuppressive therapy, studies have found that older age at transplantation was associated with an accelerated risk of post-transplant malignancy.(20,34,35) However, we did not find a significant association between malignancy and older age, male gender or diabetes mellitus. Webster et al also identified a diminished risk of post-transplant malignancy among African-Americans and Asians compared to American whites.(35)
Our study found that cytomegalovirus (CMV) seropositivity in KTX recipients had a near-significant association with cancer after transplantation. This association has also been reported by Courivaud et al,(36) who found that CMV-exposed patients had a higher cumulated incidence of cancer (30.4%), with shorter mean time to cancer occurrence than CMV-naïve patients. The exact pathogenesis was less clear, and the postulated explanations included CMV involvement in mutagenesis, inhibition of apoptosis, angiogenesis, and immunomodulation promoting malignant transformation.(36)
To date, studies on malignancy-related mortality have been limited. Our study harmonised the few publications that reported on cancer mortality rates after KTX. We have demonstrated that KTX recipients had a higher rate of mortality after diagnosis of malignancy (SMR 9.45, 95% CI 7.24–12.34), especially among male patients (SMR 18.0, 95% CI 12.51–25.91). Cheung et al(8) studied 4,895 KTX cases between 1972 and 2011, with 299 post-transplant malignancies detected in the 40,246 person-years of follow-up. In their study, the overall risk of mortality after cancer diagnosis was significantly elevated following KTX. The SMR was 2.3 (female 3.4, 95% CI 3.1–3.9; male 1.7, 95% CI 1.5–1.9), with the highest SMR found in patients with NHL (SMR 18.2).(8) A study by Farrugia et al, which analysed 19,103 KTX procedures in the UK, found that death from malignancy was the third commonest cause of death, resulting in 367 deaths and equating to a crude mortality rate of 361 malignancy-related deaths per 100,000 person-years.(37) Interestingly, Kiberd et al,(13) who analysed 164,078 first kidney-only transplant recipients from 1990 to 2004, reported an overall age- and sex-adjusted SMR of only 0.96 (95% CI 0.92–1.00). Theirs was the first study to analyse cancer-specific SMRs in kidney allograft recipients, and the authors hypothesised that this finding was a result of risk of death secondary to other causes, thus diminishing the impact of immunosuppression-induced malignancy in the older transplant cohort.(13)
The present study has some limitations. Firstly, the clinical data was retrospectively collected, although it was entered by trained investigators to ensure data completeness and accuracy. Also, our database did not capture common risk factors related to post-transplant malignancy, including cigarette smoking, alcohol consumption, family history of malignancy and, specifically, analgesic abuse, which has been reported to increase urothelial malignancies. Secondly, the follow-up period was short, which may account for this study not detecting a higher rate of post-transplant malignancies. Lastly, the modification of immunosuppression regimens occurring over time in kidney allograft recipients may have affected our ability to correlate the impact of immunosuppression and the development of post-transplant malignancy.
In summary, the present study found that KTX is associated with an increased frequency of malignancy and higher mortality in KTX recipients as compared to the general population. It also highlighted that newer immunosuppressive agents and antibody induction therapies are not significant risk factors for malignancy. However, we found the use of cyclosporine to be a significant predictor of development of post-transplant malignancies. Larger prospective studies deriving long-term follow-up from patient registries across the nation are needed in the future to give us a greater understanding of malignancy-related incidence and mortality after KTX. This study may also serve as a baseline for future comparisons with Singapore’s nationwide cancer registry in the hope of providing insights into areas of immense need for focused support and intervention. Finally, our study also underscores the implications of early detection and adoption of a more robust screening programme for developing specific strategies for cancer surveillance in KTX recipients, so as to improve the clinical outcomes of this population.
ACKNOWLEDGEMENTS
This study was presented in a poster presentation form at the 17th Congress of the European Society for Organ Transplantation on 13–16 September 2015. We are indebted to Lu York Moi and Diana Foo, transplant coordinators at SGH, Singapore, for their efforts in data collection.


