Dear Sir,
Measurement of cord blood thyroid-stimulating hormone (TSH) levels has been the primary screening strategy for congenital hypothyroidism in Singapore.(1) Screening is important, as affected infants may not present with overt clinical manifestations at birth. Prompt initiation of therapy will help to prevent long-term consequences, including intellectual disability and delayed bone maturation.(2) However, umbilical cord blood TSH levels can be difficult to interpret in babies of mothers with pre-existing or undetected thyroid conditions. Graves’ disease (GD) is a common cause of hyperthyroidism in pregnancy, affecting 0.2% of pregnant women.(3) Maternal GD has a considerable impact on neonatal outcome, depending on the interplay of changes in maternal thyroid function, presence of TSH receptor antibodies (TRAb) and use of anti-thyroid drugs (ATDs).(4) Herein, we report on two cases of babies born to mothers with hyperthyroidism to illustrate the intricacies in balancing the various factors affecting fetal-neonatal thyroid function antenatally and postnatally.
The baby in Case 1 was born at term with good weight to an apparently healthy primiparous mother. The mother was noted to have sinus tachycardia at six hours postpartum. Investigations showed that she had a raised free thyroxine (fT4) level of 26.3 (normal range [NR] 8.8–14.4) pmol/L, low TSH level < 0.010 (NR 0.65–3.70) mU/L, raised TRAb level of 4.94 (NR < 1.76) IU/L and thyroid-stimulating immunoglobulin (TSI) > 4,054% (NR 50%–179%). Notably, the mother did not exhibit other signs or symptoms of hyperthyroidism, in particular Graves’ ophthalmopathy, despite the high TSI level.(5) She was diagnosed with GD and treated with carbimazole and propranolol from postpartum Day 2. Routine cord blood screening revealed a low TSH level < 0.010 (NR 0.65–3.70) mU/L, while neonatal thyroid function on Day 4 of life showed fT4 level of 14.3 pmol/L, TSH level of 0.063 mU/L and high TRAb level of 5.20 IU/L. Subsequent testing showed low and rapidly declining fT4, with improving TSH and TRAb (
Table I
Investigation results of the babies in Cases 1 and 2.
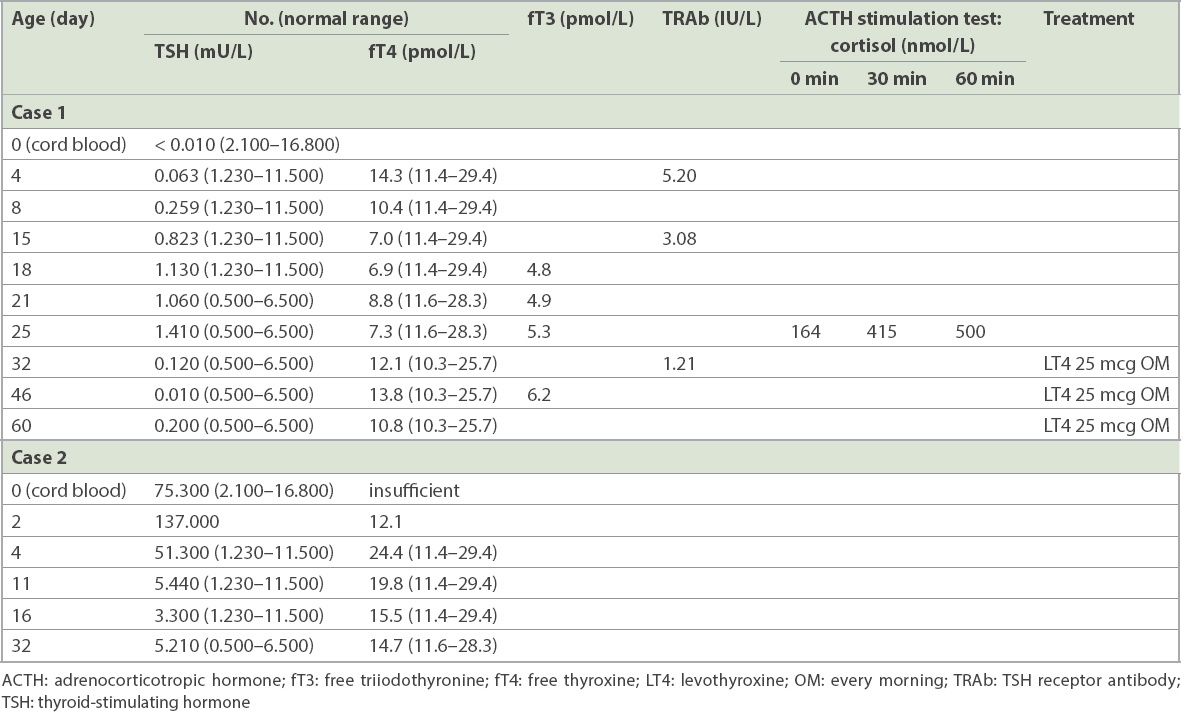
Several differentials could be considered in Case 1: (a) transient TSH suppression due to transplacental passage of maternal TSI, which could bind to the neonatal pituitary TSH receptor, resulting in suppression of neonatal TSH via a paracrine feedback loop; (b) evolving neonatal thyrotoxicosis; or (c) evolving central hypothyroidism, either from neonatal hypopituitarism or congenital central hypothyroidism in the face of prolonged fetal exposure to high maternal thyroid hormones throughout pregnancy. Without knowledge of fT4 levels at birth, it was reasonable to presume that the low cord TSH was caused by transplacental passage of maternal TSI. However, subsequent testing revealed inappropriately normal TSH with rapidly decreasing fT4 levels. When it became clear that transient TSH suppression and neonatal thyrotoxicosis were unlikely diagnoses, the next step was to exclude adrenal insufficiency through a synacthen test, prior to starting thyroxine treatment. This would avoid precipitating an iatrogenic adrenal crisis by inappropriately starting thyroxine in an infant with undiagnosed hypopituitarism. The baby was started on levothyroxine 25 mcg daily from Day 25, with normalisation of fT4 levels. Our approach was guided by repeated monitoring using several thyroid function tests in the first two weeks of life in order to narrow the range of differentials. When it became clear to us that the diagnosis in Case 1 was not primary hypothyroidism but congenital central hypothyroidism, we undertook a cautious approach of excluding potential hypopituitarism first. This would allow us to be truly certain of the diagnosis before prescribing thyroxine therapy to the baby.
The newborn achieved good growth and age-appropriate developmental milestones for the two years of follow-up. However, uncertainty remains with regard to the permanence of low thyroid function, and long-term follow-up is imperative to understand the impact of chronic exposure of the developing fetus to undetected maternal GD.
In Case 2, the baby was born at term with good weight to a healthy multiparous mother. Maternal thyroid function test in the first trimester showed a high fT4 level of 26.0 pmol/L (NR in
Table II
Investigations and treatment of the mother in Case 2.
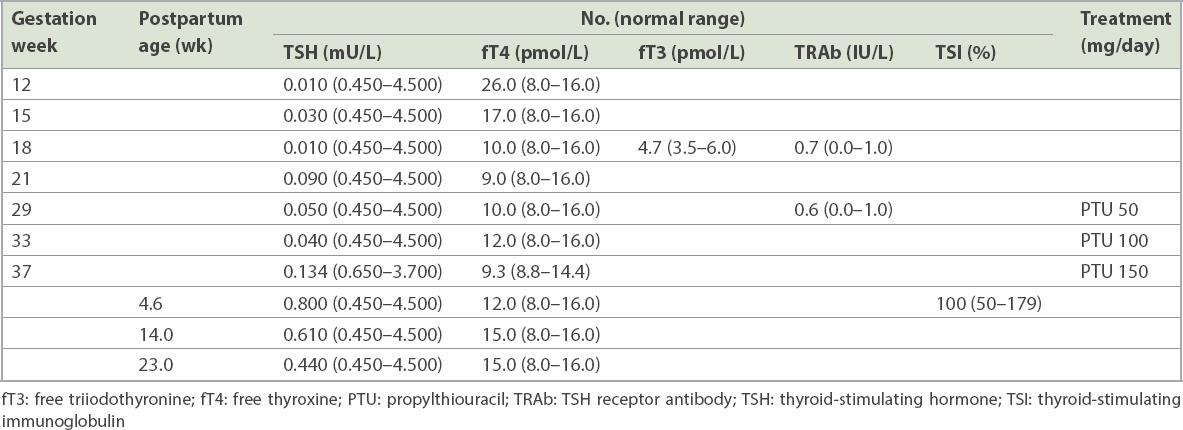
High cord TSH exceeding 40 mU/L generally triggers the ‘reflex’ initiation of thyroid replacement(7) to avoid the prospect of delayed treatment, with consequences for cognitive development.(8) However, when the maternal treatment history of escalating PTU dosages was clarified, a diagnosis of transiently oversuppressed neonatal thyroid gland from maternal ATD use became more obvious. The persistently high TSH on Day 4 of life posed a dilemma of whether thyroid replacement would be indicated or could be delayed to allow spontaneous recovery of thyroid function, knowing the effect of maternal PTU intake on neonatal thyroid status.(9) With parental understanding and compliance to close thyroid function monitoring, spontaneous recovery of the infant’s thyroid function by Day 11 led us to conclude that watchful waiting was indeed appropriate.
These two cases describe two less common presentations of babies of mothers with thyroid conditions, namely congenital central hypothyroidism and transient primary hypothyroidism. It is imperative to consider undiagnosed maternal thyroid conditions, transplacental passage of thyroid auto-antibodies, maternal anti-thyroid medications and their potential transfer during breastfeeding as factors that potentially influence neonatal thyroid function outcomes. Ideal management of such patients requires close multidisciplinary collaboration between the neonatologist, paediatric endocrinologist and adult endocrinologist for optimal outcome.


