Abstract
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is frequently associated with cholelithiasis. The prevalence of NAFLD in Asia has been on the rise, but the magnitude of this increase had not been studied previously.
METHODS
A retrospective cohort study was conducted on consecutive patients who underwent laparoscopic or open cholecystectomy from November 2001 to November 2004 (Cohort 1) and from November 2011 to November 2014 (Cohort 2) at Singapore General Hospital. Preoperative diagnostic scans (e.g. ultrasonography, computed tomography and magnetic resonance imaging) and clinical data were reviewed for the presence of fatty liver. Statistical analysis was performed.
RESULTS
In Cohorts 1 and 2, 127 patients and 99 patients were operated on, respectively. Cohort 2 had significantly higher proportions of patients with NAFLD (56.6% vs. 40.2%; p < 0.015) and hyperlipidaemia (45.5% vs. 18.9%; p < 0.001) as compared to Cohort 1. Binary logistic regression analysis showed that hypertension (odds ratio [OR] 2.558; p < 0.004) and Indian ethnicity (OR 5.448; p < 0.043) were significantly associated with NAFLD.
CONCLUSION
Similar to other international studies, we found a significant increase in the prevalence of patients with NAFLD presenting symptomatically for cholecystectomy over an interval of ten years in Singapore. Hypertension and Indian ethnicity were significantly associated with NAFLD in both time periods. This trend supports the need for concerted public health intervention to stem the increasing incidence of NAFLD and prevent its progression to more advanced liver disease.
INTRODUCTION
It has been established that non-alcoholic fatty liver disease (NAFLD) and gallstone disease (GD), or cholelithiasis, are both associated with obesity, insulin resistance, dyslipidaemia and high dietary cholesterol consumption.(1) Both conditions are also highly associated with each other and often occur comorbidly.(2) NAFLD and metabolic syndrome are proposed to be independent risk factors for GD,(3) and patients with NAFLD have a high prevalence of GD.(4) However, it remains unclear if NAFLD is a precursor of GD or whether the presence of GD is merely co-associated with metabolic syndrome that accelerates the progression of NAFLD.(1)
In Western countries, cholesterol gallstones predominate, whereas in Asia, pigment and mixed stones are more common.(5) This is interesting given that the pathogenesis of cholesterol gallstones and pigment stones are thought to share common factors related to bile metabolism.(6) Despite the possible differences in gallstone aetiology in the Asian population, NAFLD and markers of metabolic syndrome such as low high-density lipoprotein cholesterol, high serum triglycerides, high waist circumference and body mass index (BMI) are similarly associated with GD.(7,8)
We were interested in studying the risk factors for NAFLD that was comorbid with symptomatic GD in our patient population, because symptomatic patients can often be incidentally diagnosed for NAFLD during preoperative liver imaging prior to cholecystectomy. Given that NAFLD is mainly asymptomatic(9,10) and often remains undiagnosed as liver imaging is not routinely performed in the community setting, we can consider targeting symptomatic GD patients who may concurrently have NAFLD for disease management measures that are aimed at controlling the progression of NAFLD. Such measures include medical therapy and controlling the associated risk factors of NAFLD.
The current recommended management of NAFLD consists of diet control and lifestyle changes; while there is some evidence that statins, bile acid conjugates and cholesterol absorption inhibitors may be able to treat both GD and NAFLD, the efficacy of these treatments remains inconclusive.(11) A recent review on pharmacotherapies for NAFLD noted that vitamin E, pioglitazone and obeticholic acid have proven benefits, although their effect is modest.(12) Nonetheless, vitamin E supplements are still prescribed in clinics for NAFLD patients. If left unreversed, NAFLD may progress to more advanced liver diseases such as liver cirrhosis and hepatocellular carcinoma.(4)
The prevalence of NAFLD varies across different populations and is increasing globally.(13) The city-state of Singapore has a multiracial population that reflects that of Southeast Asia. In Asian populations, non-obese patients reportedly have a greater tendency to develop NAFLD compared to Western populations.(14) Reports have also suggested a rise in the prevalence of NAFLD in Asia, affecting up to 30% of the general population, although the associated obesity and diabetes pandemics are thought to have occurred later than in the West.(15,16) However, there is a dearth of literature that objectively compares multiple cohorts from the same patient population at different time points. Hence, we were also interested in characterising the magnitude of the increase in the prevalence of NAFLD among local symptomatic GD patients from an epidemiological perspective.
Accordingly, the aim of this study was to establish the relative prevalence of cholelithiasis-associated NAFLD and identify the risk factors associated with NAFLD in patients who presented symptomatically for cholecystectomy over two cohorts spaced ten years apart in a Southeast Asian population with BMI stratification. Changes in the patient pool were likely to be representative of changes in the general population. We hypothesised that there would be an increase in the prevalence of NAFLD in accordance with international trends, with a null hypothesis that there would be no significant increase over ten years.
METHODS
A retrospective cohort study was performed on consecutive patients who underwent laparoscopic or open cholecystectomy at Singapore General Hospital, Singapore, over two time periods separated by ten years, namely between November 2001 and November 2004 (Cohort 1) and between November 2011 and November 2014 (Cohort 2), and who fulfilled the inclusion and exclusion criteria (
Box 1
Inclusion and exclusion criteria for patient enrolment:
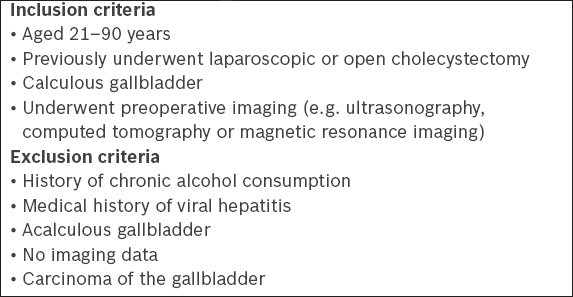
Box 2
Demographic, medical and lifestyle characteristics retrieved:

Reports of diagnostic imaging, such as ultrasonography, computed tomography or magnetic resonance imaging, were reviewed for the presence of fatty liver disease. If multiple modalities were used, a positive report was taken to be true. If there was no report of fatty changes to the liver, the result was taken to be negative. This study was approved by the SingHealth Centralised Institutional Review Board (CIRB Ref. No.: 2015/2035).
Chi-square test was used to analyse whether it was by chance that any change occurred in the proportion of patients with NAFLD, as well as other medical comorbidities and demographic characteristics between the two cohorts, while a two-tailed Student’s t-test (a = 0.05) was used to compare for differences in mean age and BMI. Univariate binary logistic regression was performed on all factors to be analysed, and factors with p < 0.2 were included in the multivariate logistic regression model. Hosmer-Lemeshow goodness-of-fit test was used to check for adequacy of fit during multivariate logistic regression analysis. Statistical analysis was performed using IBM SPSS Statistics version 20.0 (IBM Corp, Armonk, NY, USA). A p-value < 0.05 was considered to be statistically significant.
RESULTS
A total of 226 patients fulfilled the study criteria: 127 patients were from Cohort 1 (surgery in November 2001–November 2004) and 99 patients were from Cohort 2 (surgery in November 2011–November 2014). The demographic profiles of the two cohorts were largely similar, except for a decrease in the proportion of Chinese patients over time (p < 0.006). Accordingly, multivariate adjustment was performed to account for ethnicity in the subsequent logistic regression analysis.
Table I
Demographic characteristics of patients.
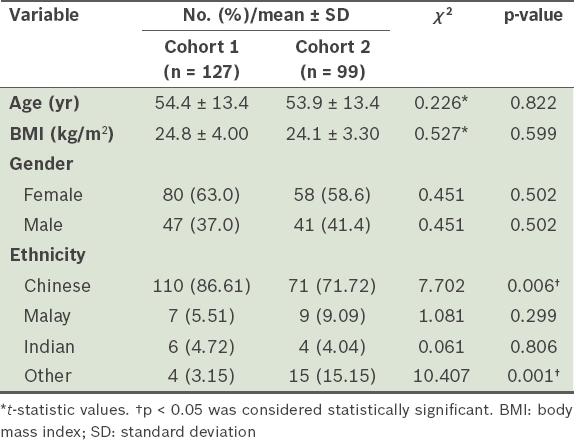
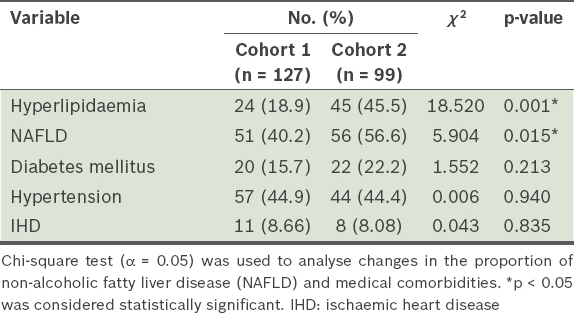
We found that Cohort 2 had a significantly higher proportion of patients with NAFLD (56.6% vs. 40.2%, p < 0.015) and hyperlipidaemia (45.5% vs. 18.9%, p < 0.001) as compared to Cohort 1 (
Table II
Comorbidities of patients from both study cohorts.
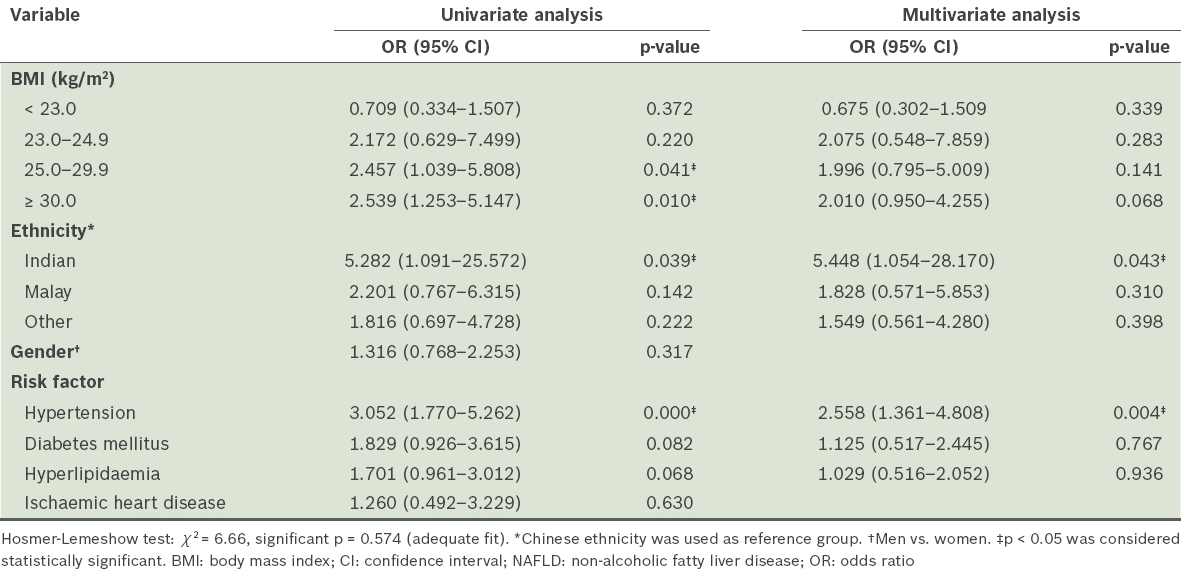
As the Asian population presents with obesity at lower BMI ranges,(17,18) the definitions of overweight, obesity and severe obesity were stratified in our study according to World Health Organization guidelines for the Asia-Pacific region, as follows: normal (BMI 18.5–22.9 kg/m2); overweight (BMI 23.0–24.9 kg/m2); obese (BMI 25.0–29.9 kg/m2); and severely obese (BMI ≥ 30.0 kg/m2).(19) Logistic regression analysis of possible risk factors revealed that hypertension (odds ratio [OR] 2.558; p < 0.004) and Indian ethnicity (OR 5.448; p < 0.043) were factors that were positively associated with NAFLD in our study after multivariate adjustment for ethnicity, BMI and medical comorbidities (
Table III
Logistic regression analyses of medical and demographic variables, with NAFLD as the dependent variable.
DISCUSSION
Over the ten-year interval, there was a significant increase in both NAFLD and hyperlipidaemia in patients who underwent cholecystectomy at our centre. NAFLD was significantly associated with hypertension, BMI and Indian ethnicity. Despite the significant increase in the proportion of patients with hyperlipidaemia, and some degree of association between hyperlipidaemia and NAFLD in the univariate analysis (OR 1.701; p < 0.068), we found no significant association after multivariate adjustment. This contrasts with another multiethnic Asian study performed on a general population pool in which hypertriglyceridaemia and low high-density lipoprotein were found to be independent predictors in a multivariate model,(20) and with the wider international literature, which shows a strong association between hyperlipidaemia and NAFLD.(21) The discrepancy could be due to our smaller sample size and the fact that our study only included patients with GD. As GD is known to be associated with hyperlipidaemia and other manifestations of metabolic syndrome,(22) the significance of hyperlipidaemia may have been negated as an independent risk factor in our patient pool.
However, our study concurs with the established literature that arterial hypertension, a manifestation of metabolic syndrome, is independently associated with NAFLD. It was previously found that the prevalence of NAFLD in non-obese and non-diabetic hypertensive patients was at least twice that of the general population.(23) Our results indicate that hypertension, in addition to BMI, needs to be better controlled in our local population.
In the present study, BMI stratification showed that overweight and non-severely obese patients may be at increased risk for NAFLD, although this association did not reach statistical significance. However, it is worth noting that the risk of developing NAFLD increased as we progressed up the BMI strata. Overweight patients with BMI of 23.0–24.9 kg/m2 (OR 2.075; p < 0.283) and obese patients with BMI of 25.0–29.9 kg/m2 (OR 1.996; p < 0.141) demonstrated increasing ORs and statistical significance. These findings were similar to those of another multiethnic Asian study, which looked at patients with BMI > 23.0 kg/m2 as a group and found that their BMI was significantly associated with NAFLD.(20) Our study, which used a greater degree of stratification, does not directly concur with the finding that NAFLD among non-obese persons (i.e. those with BMI 23.0–24.9 kg/m2) is prevalent in the Asian population.(14) Instead, our results indicate that clinicians should target patients in the higher BMI groups and graduate management accordingly. These findings can be useful in establishing BMI targets for the purposes of weight management in the local population.
Although our study showed that Indian patients were at greater risk for NAFLD when compared to Chinese patients, we noted that the confidence interval was very wide because the sample of Indian patients in our study was small (n = 10). However, this result is in line with regional studies showing that patients of Indian ethnicity are more predisposed to NAFLD than Chinese patients, ostensibly due to the higher prevalence of obesity in the former group.(20,24) Nonetheless, having Indian ethnicity as an independent risk factor in our multivariable model supports the notion that there are likely other genetic predispositions in this ethnic group. Interestingly, it was previously shown that Asian Indians have a higher body fat percentage and abdominal adiposity even when BMI is within normal limits.(25)
Regarding the general increase in NAFLD prevalence over the years, a possible cause may be lifestyle or diet choices. For example, the dietary consumption of fructose has been linked to NAFLD, given that fructose is metabolised by the liver.(26) As fructose is commonly added to fruit juice and sweet drinks, it is a suitable candidate for dietary control measures. One could also postulate that in Singapore, an increasingly Westernised diet, sedentary lifestyles, readily accessible fast foods and increased calorie intake could be contributing factors for this change in prevalence.(15) However, there is a dearth of detailed primary studies in the recent literature that would help us to identify specific lifestyle factors behind this shift.
We acknowledge that there are several limitations to this study. The radiological reporting may not have been consistent, as this was a retrospective study, and the sensitivity of diagnostic imaging may have changed over time. As there was no specific request to report fatty liver disease, our results were likely to be conservative, even though all abnormalities, including incidental findings, were to be reported as part of routine protocol. Patient history on chronic alcohol consumption was taken from preoperative anaesthesia notes and may not have specified the exact amount consumed, although chronic alcohol consumption is ideally defined as alcohol consumption of more than 20 g daily (or 140 g weekly) for men and more than 10 g daily (or 70 g weekly) for women.(16) Finally, we were limited to data on NAFLD from a subset of patients with symptomatic GD, which we assumed was in accordance with trends in the general population from an epidemiological standpoint. Nonetheless, such data was still useful for our objective of proactively identifying patients for further management.
In conclusion, at our centre, there was a significant increase in the proportion of patients with symptomatic cholelithiasis comorbid with NAFLD and hyperlipidaemia over the span of ten years, which is alarming from an epidemiological perspective. Hypertension and BMI > 30.0 kg/m2 are the main factors that require more aggressive control in our patient population, so as to stem the increasing incidence of NAFLD before it progresses to more advanced liver disease. These results allow us to take steps to plan confirmatory studies to establish the characteristics of individuals who are likely to be at risk and also help to identify at-risk patients for future prospective trials.
ACKNOWLEDGEMENT
The authors would like to thank Ms Phang Su Ting, Research Executive, Department of General Surgery, Singapore General Hospital, Singapore, for her support in research administration.


