Abstract
INTRODUCTION
Intravesical Bacillus Calmette-Guerin (BCG) therapy is the standard adjuvant treatment for non-muscle-invasive bladder carcinoma (NMIBC) with carcinoma in situ, in addition to tumour resection. We aimed to study BCG complications that preclude adequate treatment of NMIBC in an Asian population.
METHODS
This retrospective study was conducted using a large, prospectively maintained bladder cancer database. 336 patients received intravesical BCG therapy for bladder cancer in our institution between 2004 and 2016, with an average follow-up duration of 63 months.
RESULTS
The study included 258 (76.8%) male and 78 (23.2%) female patients. The median age of the patients at diagnosis of bladder cancer was 69 (range 17–94) years, and the median number of BCG instillations was 6 (range 1–27). 52 (15.5%) patients received maintenance therapy. The most common complications included urinary tract infection with/without sepsis (n = 18, 5.4%), haematuria (n = 9, 2.7%) and acute urinary retention (n = 4, 1.2%). 93.3% of the patients with complications presented early, within one month of completion of therapy. 22 out of 30 complications were Clavien-Dindo grade ≤ 2. 10 (33.3%) patients were admitted to hospital because of BCG-related adverse effects. The most common reasons for termination were urosepsis (2/30, 6.7%) and acute urinary retention (2/30, 6.7%). Patients aged ≥ 80 years at diagnosis were at higher risk of developing BCG-related complications (19.0% vs. 7.5%, p = 0.01).
CONCLUSION
This retrospective cohort and subgroup study showed that intravesical BCG therapy is well tolerated and has a low incidence of complications even in the elderly and patients with multiple comorbidities.
INTRODUCTION
Intravesical Bacillus Calmette-Guerin (BCG) therapy is the standard adjuvant treatment for non-muscle-invasive bladder carcinoma (NMIBC) with carcinoma in situ, in addition to tumour resection. Morales et al first described intravesical BCG treatment of NMIBC in 1976.(1) BCG is an attenuated strain of Mycobacterium bovis with minimal virulence. The exact mechanism of intravesical BCG is still unknown. However, it has been found to elicit a local immune response against tumour cells, accompanied by a release of tumour necrosis factors.(2,3) Although BCG is generally well tolerated in immunocompetent patients, it has the potential to produce local and systemic side effects, as it is a live (though attenuated) bacterium. We aimed to study the complications of intravesical BCG that precluded adequate treatment of NMIBC in an Asian population.
METHODS
This Institutional Review Board-approved retrospective study was conducted using data from a large urology cancer registry established in 1988 in a single tertiary academic institution. Electronic medical records were available only from 2004. In order to minimise missing data, we also evaluated patients from 2004 to 2016; 336 patients who received intravesical BCG (both induction and maintenance therapy) during this time frame were included. Information obtained from patients’ electronic health records included demographics, comorbidities, histopathology reports, treatment descriptions (such as surgery and BCG administration regime) and long-term outcomes.
Over the years, our pharmacy has carried the following intravesical BCG preparations: BCG 12.5 mg freeze-dried (OncoTICE®, Merck Sharp & Dohme Ltd, Hertfordshire, United Kingdom), BCG 81 mg Connaught strain (ImmuCyst®, Sanofi Pasteur, Ontario, Canada) and BCG freeze-dried 80 mg Vac. Each of these preparations was mixed in 50 mL of preservative-free saline and introduced intravesically via a Nelaton catheter. Care was taken to perform atraumatic catheterisation. The dwell time of BCG was generally two hours. Instillations were initiated 2–3 weeks after transurethral resection of bladder tumour to allow healing of the urothelium. Patients received a weekly dose of induction treatment for six weeks. Maintenance therapy was prescribed by the attending urologists according to European Association of Urology guidelines.
Adverse events documented by the attending physicians were captured. For the purpose of this study, a clinically significant BCG complication is defined as one that resulted in termination of therapy, medical intervention and/or hospitalisation. Mild bladder or generalised flu-like symptoms that were not sufficiently severe to prompt discontinuation of therapy or additional medical treatment were not considered significant complications in this analysis. Out of 336 patients, 30 (8.9%) were identified to have clinically significant BCG complications. A subgroup analysis of these 30 patients was conducted.
Chi-square test was used for categorical variables and Student’s two-tailed t-test was used for continuous variables. Statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp, Armonk, NY, USA), and p < 0.05 was considered significant.
RESULTS
The study included 258 (76.8%) male patients and 78 (23.2%) female patients, with an average follow-up duration of 63 months. The median age of the cohort at the initiation of BCG treatment was 69 (range 17–94) years. Of these, 83.6% were Chinese, 4.5% Malay, 3.6% Indian and 8.3% of other ethnicities. 80% of the patients had a Charlson Comorbidity Index score of ≤ 4. The median number of BCG instillations was 6 (range 1–27;
Table I
Demographic, tumour and treatment characteristics of patients who received intravesical Bacillus Calmette-Guerin (n = 336).
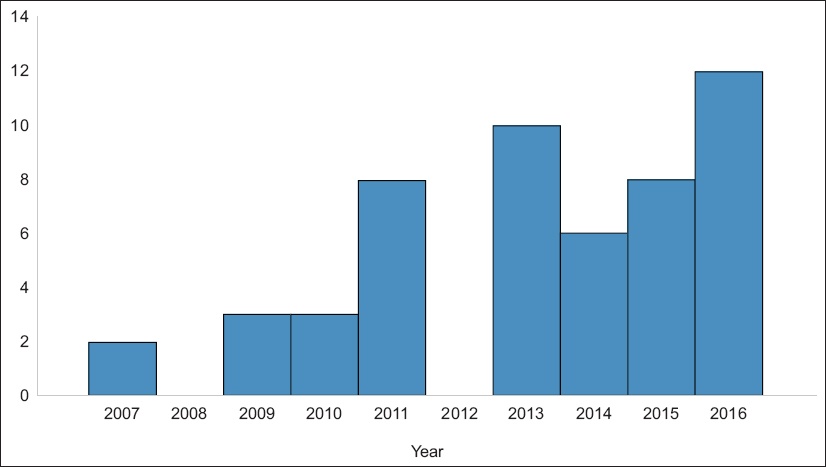
Fig. 1
Graph shows the number of maintenance Bacillus Calmette-Guerin therapies by year.
In all, 30 patients developed BCG-related complications. The most common complications (
Table II
Clinically significant Bacillus Calmette-Guerin (BCG)-related complications in the cohort (n = 336) and treatment outcome of patients with these complications (n = 30).
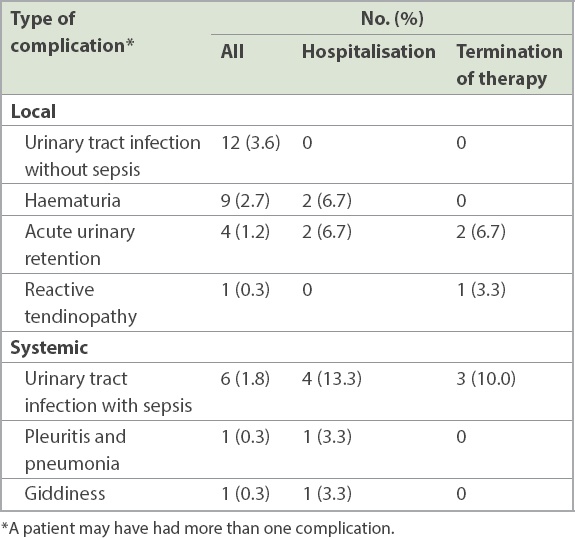
Out of the 30 patients with complications, 7 (23.3%) had Grade 3 complications, according to the World Health Organization’s grading of toxic drug effects.(4) One patient developed Grade 4 complications (pleuritis and pneumonia), while the rest had Grade 2 complications. 10 (33.3%) patients had to be admitted to hospital because of BCG-related adverse effects. There were no mortalities associated with BCG complications. Therapy was terminated in 16 (53.3%) patients before the completion of the intended duration of therapy – of these, 14 (87.5%) patients stopped treatment before or upon completion of six weeks of induction therapy. The most common reason for termination was urosepsis (2/30, 6.7%), acute urinary retention (2/30, 6.7%) and reasons unrelated to BCG therapy (2/30, 6.7%) such as defaulting of follow-up visits. Other reasons included rash (1/30, 3.3%), fever (1/30, 3.3%) and arthritis (1/30, 3.3%).
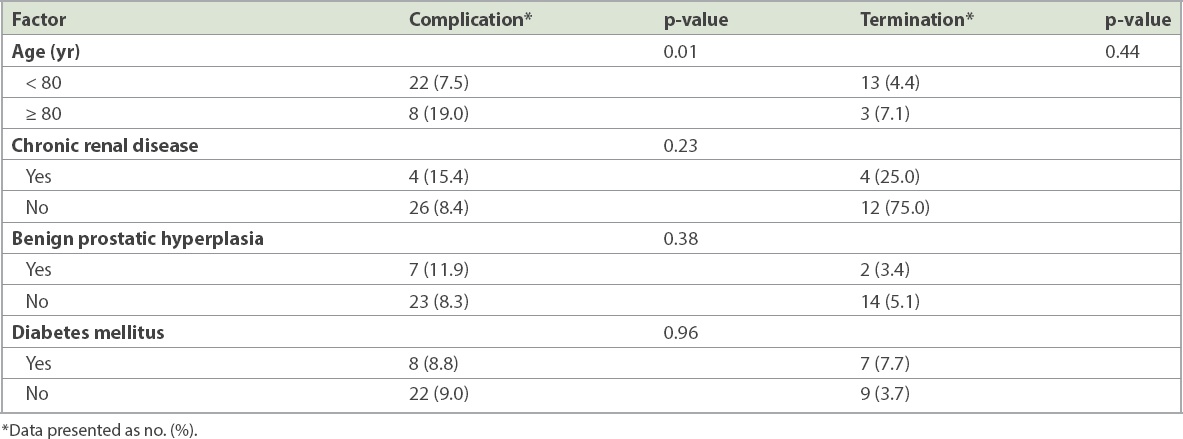
Demographic characteristics such as gender (p = 0.36), race (p = 0.57) and Charlson comorbidity index score (p = 0.12) were not associated with increased complication rates. Age was analysed as a continuous variable and as a category. As a continuous variable, age was not associated with an increased rate of complications (p = 0.48). However, patients aged ≥ 80 years at diagnosis were at higher risk of developing BCG-related complications (19.0% vs. 7.5%, p = 0.01; odds ratio 2.90, 95% confidence interval 1.20–7.02). However, these older patients were not at higher risk of treatment termination (7.1% vs. 4.4%, p = 0.44). A greater proportion of patients with chronic renal disease (15.4% vs. 8.4%, p = 0.23) and benign prostatic hyperplasia (11.9% vs. 8.3%, p = 0.38) developed complications, but this was not statistically significant. Complications were equally common in patients with and without diabetes mellitus (8.8% vs. 9.0%, p = 0.96;
Table III
Patient factors associated with the risk of Bacillus Calmette-Guerin-related complications and therapy termination.
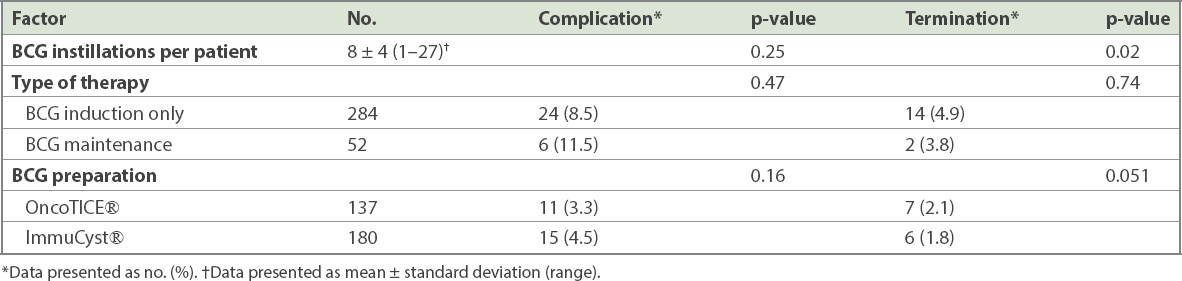
The occurrence of complications did not increase with the number of BCG instillations (p = 0.25). 40% of patients who had complications developed them during or shortly after six doses of induction BCG. The three BCG preparations used, OncoTICE, ImmuCyst and freeze-dried Vac, were comparable in terms of complication rates (3.3% vs. 4.5% vs. 1.2%; p = 0.16) and termination of therapy (2.1% vs. 1.8% vs. 0.9%; p = 0.051;
Table IV
Bacillus Calmette-Guerin (BCG) factors associated with risk of BCG-related complications and therapy termination.
To complete the picture, we also looked at the recurrence and progression rates for all 336 patients. Of the 16 patients who did not complete induction therapy, 8 (50.0%) had recurrence of disease (p = 0.74) and 1 (6.3%) had progression of disease (p = 0.73). 13 (81.3%) patients had high-risk disease and 3 (18.7%) had intermediate disease. 7 (43.6%) patients had neither recurrence nor progression of disease. Of the 320 patients who continued with therapy, 131 (40.9%) had disease recurrence, 32 (10.0%) had progression of disease and 157 (49.0%) had neither recurrence nor progression of disease. There were no statistical differences in recurrence (p = 0.24) and progression (p = 0.28) rates between the two groups.
DISCUSSION
NMIBC is diagnosed in about 70% of all newly diagnosed bladder cancer cases. It has a high recurrence rate of 40%–80%,(5,6) with less than 15% of NMIBC cases progressing to muscle-invasive or metastatic disease.(7) The first-line gold standard treatment for NMIBC is intravesical BCG to reduce the risk of recurrence and progression.
BCG-mediated local immune response in the bladder results in inflammation and anti-tumour activity.(8) Therefore, an intact immune system is essential for intravesical BCG therapy to be effective.(9,10) BCG instilled into the bladder allows the tumour cells to take on the antigenic features of BCG-infected phagocytes, which initiate an inflammatory cascade that ultimately leads to the elimination of tumour cells.(8) Not surprisingly, the same local immune response can result in local side effects. The incidence and severity of toxicity have been extensively studied.(11-13) Witjes et al(12) reported cystitis and haematuria in 80% and up to 90% of patients, respectively. Granulomatous prostatitis is a common occurrence after BCG therapy; however, it is usually asymptomatic, with local and systemic reactions occurring in only 1%–3% of patients. Rischmann et al(13) reported that the incidence rate of epididymo-orchitis can be as low as 0.2%. The most common systemic side effects associated with BCG therapy are general malaise, fever, myalgia and nausea. Lamm et al reviewed 2,602 treated patients and found that less than 5% had serious adverse events.(14) However, local and systemic side effects may lead to the termination of intravesical BCG treatment in approximately 20% of patients.(15) The available literature is mostly from Western countries, while the larger, more comprehensive studies were published before 1995. Recent studies consist mostly of reports of a singular case. In our study, we focused on clinically significant complications that led to termination of therapy, additional medical therapy and/or hospitalisation. Our results demonstrated that intravesical BCG therapy is well tolerated, with only a small proportion of patients developing severe adverse events (8.9%), which is comparable to that reported by previous studies.
Of note, termination of BCG therapy likely did not affect disease outcome. Our overall rates of recurrence (139/336, 1.3%) and progression (33/336, 9.8%) are comparable to and remained lower than reported numbers.(7-9) However, the number of treatment terminations may be too small to draw a conclusion.
Reactive arthritis occurs in 0.5%–1% of bladder cancer patients treated with intravesical BCG.(16) 60% of these subjects are positive for HLA-B27 allele,(17) seronegative for rheumatoid factor and antinuclear antibodies, and have a transient course that is rarely chronic and responds to treatment with anti-inflammatory drugs, glucocorticoids, anti-tuberculosis drugs and/or discontinuation of immunotherapy.(18) Raheem et al published a review of 23 case reports and four review articles from January 1986 to January 2011. A total of 40 patients were studied. Knees (41%), ankles (26%) and wrists (19%) were the most commonly affected joints. Most patients developed symptoms after the fourth instillation of BCG (25%).(16) One of our patients, who was found to be HLA-B27-positive, developed symptoms after her fifth dose of BCG. Her symptoms resolved with anti-inflammatory drugs and within seven months of ceasing BCG therapy.
Gonzalez et al stratified BCG infections that occurred after intravesical therapy into early- and late-presentation disease.(19) Early-presentation disease results from systemic infection of immunologically competent hosts by a pathogen of low virulence (such as BCG). These patients typically present within three months of BCG treatment. Late presentation-disease results from reactivation of infection after successful immunologic control of early dissemination. Granulomas are uniformly present, and these were observed on histological examination of the pleural biopsy of our patient who developed pleuritis and pneumonia 18 months following BCG therapy. Mycobacterium tuberculosis complex was isolated on tissue culture. Efforts should be made to differentiate M. bovis from M. tuberculosis, because tuberculosis is prevalent in our population and reactivation of latent tuberculosis should be excluded.
Our results showed that the majority of patients who discontinued therapy owing to the adverse effects of BCG did so during induction therapy. Maintenance therapy did not result in increased rates of complications or therapy termination. This is consistent with the rates reported in a study of 487 patients who received BCG, where one-third of the treatment terminations occurred after six months of therapy.(20) A literature review and a single-institution series of 282 patients found no differences in the number of BCG instillations between those with and without BCG-related complications.(21) Therefore, maintenance therapy should be considered in patients who tolerate induction therapy without developing complications.
In 2007, Heiner and Terris reported that patients with BCG-related complications were significantly older than those without complications (mean age 76.0 years vs. 70.3 years; p < 0.00001), and that the complication rates of patients aged ≥ 70 years were similar to those observed in patients aged ≥ 80 years (48.6% vs. 46.2%).(22) In our patient population, only patients who were older than 80 years were found to have a statistically significantly increased risk of developing severe complications. The slight discrepancy could be due to the inclusion of female patients in our study group unlike in the Heiner study, which evaluated only male veterans. Joudi et al proposed that ageing is associated with decreased response to intravesical immunotherapy, and this is particularly apparent in patients older than 80 years.(23) Therefore, the risks and benefits of intravesical BCG therapy should be weighed carefully in older patients, given that they have a significantly higher risk of developing severe BCG complications, and the efficacy of the intravesical therapy may be decreased in this population. Therefore, immune response and toxicity should be monitored, and treatment should be individualised.
The predisposing factors for developing BCG infection after intravesical instillation are not yet well defined. There have been concerns regarding the potential for systemic BCG infection in immunocompromised patients. However, many studies have shown that immunosuppression is not associated with increased risk for severe BCG-related complications.(24-26) Similarly, our study did not find any significant association between comorbidities (such as chronic renal disease and diabetes mellitus) and the risk of developing complications from intravesical BCG therapy.
Owing to the shortage of BCG, various strains and preparations were used over the years in our institution. However, we found no association between a particular strain and the risk of complications or therapy termination. This is supported by a meta-analysis that could not decipher any differences among BCG strains.(27)
There are several limitations to this study. 49 (14.6%) of our patients defaulted follow-up during the study period, but we were unable to ascertain the reasons for the default. Therefore, the rate of complications may have been higher than what was reported in this study. Furthermore, owing to the rarity of severe BCG complications, it was difficult to obtain a large enough patient population, and thus, we should endeavour to carry out a multicentre prospective study in the future.
In conclusion, this retrospective cohort and subgroup study showed that intravesical BCG therapy is well tolerated and has a low incidence of complications, even for patients with multiple comorbidities. The number of instillations does not affect the risk of clinically significant complications. The findings of this study suggest that BCG should be used with caution in elderly patients, and patients older than 80 years of age should be monitored closely for BCG-related complications.


