In an article recently published in the Singapore Medical Journal, Wong et al compared the clinicopathological patterns and survival outcomes of colorectal cancer (CRC) between patients with young- and late-onset CRC in Malaysia.(1) Young onset was defined as an age of less than 50 years, the current age threshold to begin CRC screening in asymptomatic average-risk adults. The proportion of young-onset CRC was 10.7%, and 76.1% of the affected patients had no known risk factors. In both age groups, CRC was mostly diagnosed at an advanced stage. Young-onset CRC had more aggressive tumour characteristics.(1)
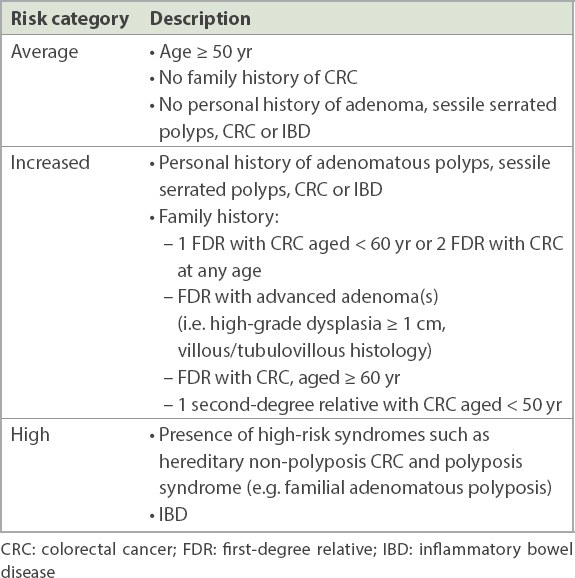
In Singapore, CRC is the most common cancer in men and the second most common cancer in women.(2) CRC screening reduces the incidence of CRC and CRC-related mortality. Detection and resection of adenomatous colonic polyps interrupts the adenoma-carcinoma sequence. Endoscopic resection may be curative for intramucosal carcinoma without high-risk features. A risk-stratified approach is adopted for CRC screening, as shown in
The American College of Gastroenterology and the United States (US) Preventive Services Task Force published updated CRC screening guidelines in 2021. CRC screening for all average-risk adults aged 50–75 years remains strongly recommended, as there is convincing evidence that screening can accurately detect early-stage CRC and adenomatous polyps. These guidelines now also recommend screening for average-risk adults aged 45–49 years.(5,6) A rising incidence rate of CRC has been observed among young adults in the US and other developed Western countries. This has been attributed to a birth cohort effect, with the increased risk of CRC of those born after 1950 being carried forward as they age. This increase in risk is multifactorial, and the Western lifestyle has been implicated.(7) Microsimulation modelling suggests that for average-risk adults in the US, starting CRC screening at 45 years of age provides an efficient balance between colonoscopy burden and life-years gained.(8)
In this context, it is important to objectively assess whether this new US recommendation to commence CRC screening from 45 years of age in average-risk adults is relevant to Singapore. Different populations have intrinsic differences in patient characteristics and healthcare systems. Population-level screening requires good and robust evidence that a test is clinically effective and cost-effective for screening of the general population. Conversely, screening may be suitable only for individual-level decision-making, such as being useful for high-risk populations, or when there is evidence that the test is clinically effective but its cost-effectiveness has not been evaluated or is not favourable.
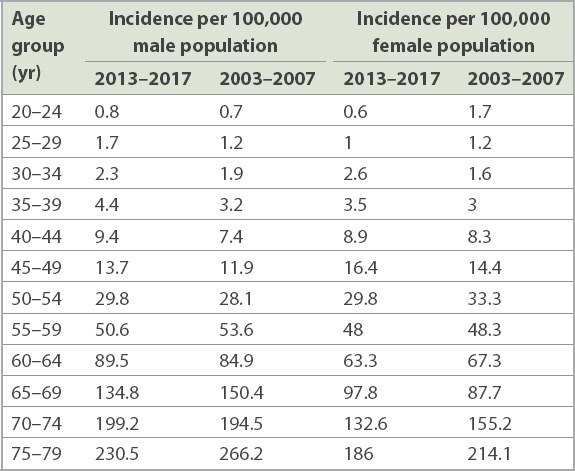
Singapore’s national CRC screening programme was launched by the HPB in 2011. It is necessary to evaluate whether there has been a significant increase in the CRC incidence rate in average-risk young adults since then, and if so, whether a systematic screening programme is cost-effective when screening begins at 45 years of age. There is now greater awareness of the benefit of CRC screening in Singapore, and it is not unusual for average-risk individuals to directly request for a screening colonoscopy, even in those aged younger than 50 years. Data concerning the incidence of CRC in Singapore in two five-year periods (2003–2007 and 2013–2017), stratified by age based on published data from the Singapore Cancer Registry, is summarised in Tables
Table III
Incidence rate of rectal and rectosigmoid cancer by five-year age groups: 2003–2007 vs. 2013–2017.(9)
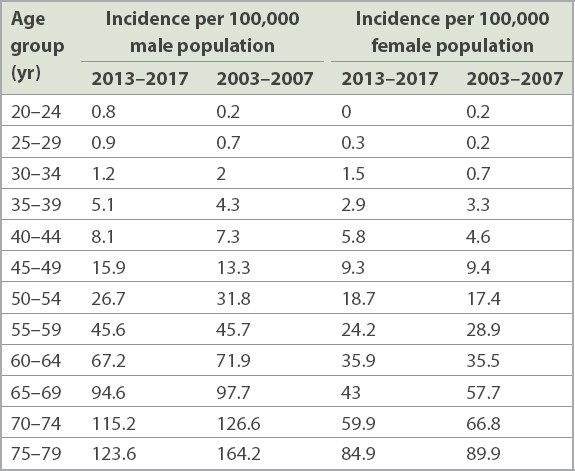
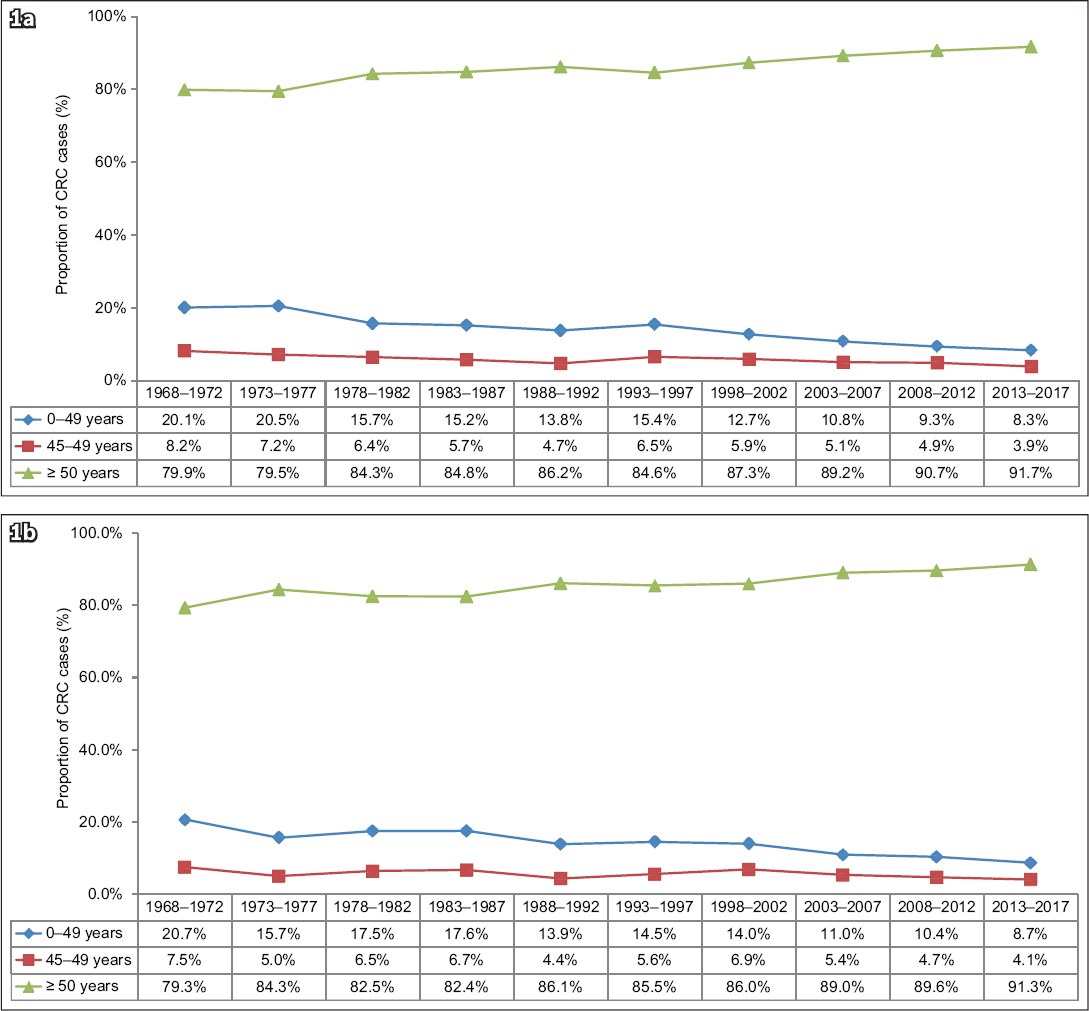
Fig. 1
Charts show the proportion of colorectal cancer in (a) men and (b) women in Singapore by age group, in five-year time periods from 1968–1972 to 2013–2017.(9) CRC: colorectal cancer
It is premature to advocate for starting population-based CRC screening of average-risk adults at 45 years of age in Singapore. Efforts should be focused on further improving CRC screening take-up rates in the current programme. Nevertheless, the situation may change with time, in terms of CRC incidence rate and cost-effectiveness, and this issue will need to be periodically reviewed. CRC screening can be performed for asymptomatic average-risk individuals aged 45–49 years who are concerned about CRC; however, they should be advised on the rationale of current recommendations and that screening will be performed outside of the current national screening framework. Symptomatic individuals should undergo timely diagnostic evaluation.