Dear Sir,
We herein report an unusual case of ectopic phaeochromocytoma arising from an accessory adrenal gland, which was incidentally discovered during laparoscopic surgery when bilateral adrenalectomy was performed for phaeochromocytomas in a patient with multiple endocrine neoplasia Type 2A (MEN-2A). This observation has important implications for surgical considerations when bilateral adrenalectomy is contemplated in MEN-2A cases.
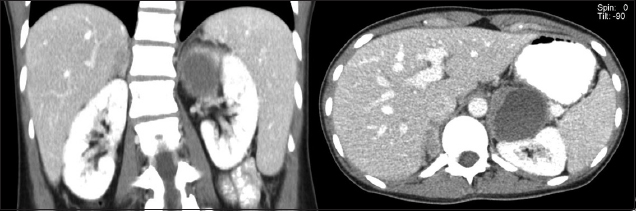
A 25-year-old woman presented with anterior neck swelling. She was clinically and biochemically euthyroid. She had markedly elevated calcitonin levels of 690 (normal range [NR] 0–10) ng/L and fine needle aspiration of both thyroid nodules showed features of medullary carcinoma. RET proto-oncogene mutation studies confirmed the diagnosis of MEN-2A. Although the patient was normotensive, her 24-hour urine catecholamine excretion values were markedly elevated (metanephrine 10,420 [NR 264–1,729] nmol/day, normetanephrine 9,864 [NR 480–2,424] nmol/day). Computed tomography of the abdomen revealed a 1.4 cm × 1.2 cm × 1.3 cm right adrenal nodule and a 4.7 cm × 5.3 cm × 5.8 cm heterogeneous cystic nodule in the left adrenal gland (
Fig. 1
CT images show the bilateral adrenal nodule.
During the resection of the left adrenal gland, a small, pale brown nodule (1.3 cm × 1.2 cm × 0.4 cm in size) was noted over the left renal vein (
Fig. 2
Photograph shows laparoscopic view of the accessory adrenal gland (circled).
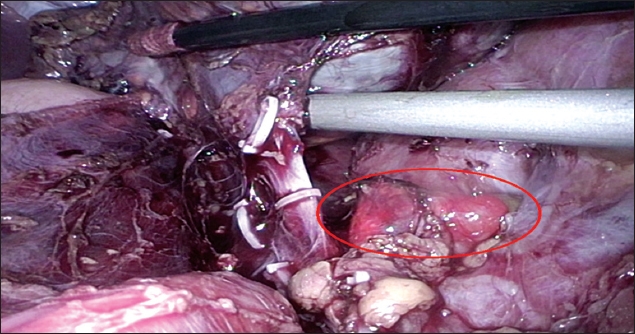
Fig. 3
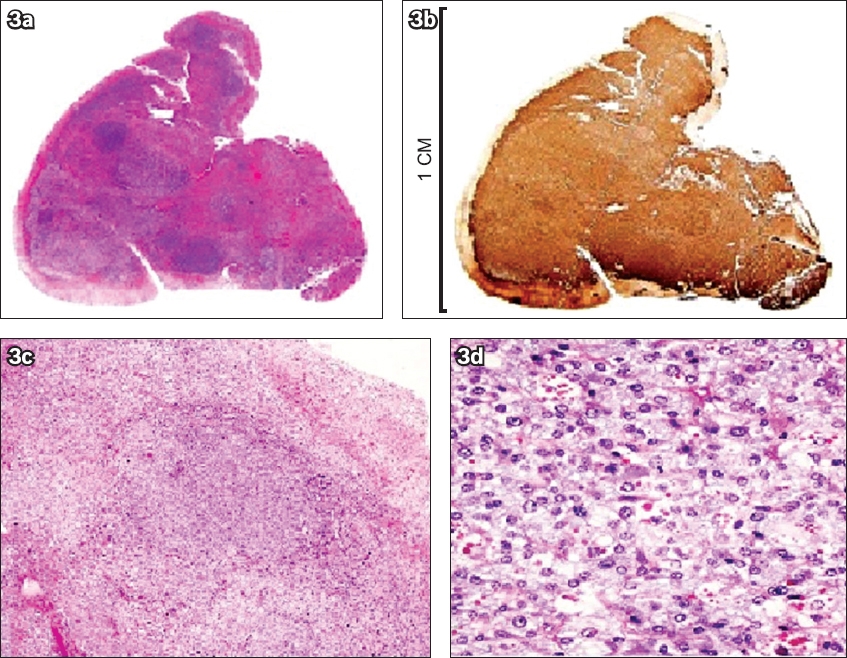
Histology and immunohistochemistry of the accessory adrenal gland. Photographs show (a) whole nodule and (b) chromogranin immunostain of the peripheral unstained cortex. Photomicrographs show (c) expanded medulla (Haematoxylin & eosin, × 40) and (d) nested architecture and hyaline cytoplasmic globules (Haematoxylin & eosin, × 200).
The lifetime risk of developing phaeochromocytomas in patients with MEN-2A has been estimated at 50%,(1) although large variability has been reported depending on the specific RET germline mutation. The dual embryological origin of the adrenal glands from the mesoderm and ectoderm, as well as embryological migration, allows the potential formation of accessory adrenal glands. It has been postulated that ectopic or accessory adrenal tissue occurs when fragments of tissue break off during development. The break-off point at migration may therefore determine if the ectopic tissue may or may not contain medullary tissue; the more proximal fragmentation point may contain medullary tissue, while the more distal fragmentation point may only contain cortical tissue associated with the descending gonads.(2) MEN-2A patients are known to have high prevalence of phaeochromocytomas and a high propensity to develop these over time. Hyperplastic and neoplastic cells are the precursor cells for bilateral MEN-2 phaeochromocytomas, and were described in 63% of MEN-2 patients in a recently published large, multinational, observational, retrospective population-based study.(3)
Very few cases of pheochromocytoma arising from accessory adrenal glands have been reported in literature. This has been partly explained by the low occurrence rate of medullary tissue in ectopic adrenal glands, which was estimated to be 16% in autopsy studies.(2) An accessory adrenal gland phaeochromocytoma is extremely rare in a MEN-2A case, with only three such case reports in literature.(4,5)
Accessory adrenal glands can be the cellular basis for phaeochromocytoma, and it is therefore important to emphasise continual follow-up for phaeochromocytoma in subjects with MEN-2A, even after bilateral adrenalectomy. Awareness of the existence of aberrant adrenal tissues and the embryological migration of adrenal medullary tissue is of clinical significance, as these tissues may undergo compensatory hypertrophy and result in neoplasms, making it difficult to achieve surgical goals.
Yours sincerely,


