Abstract
INTRODUCTION
There are many treatment options for vestibular schwannomas (VSs), including radiosurgery. Previous studies have shown good outcomes for smaller tumours. We report the results of a seven-year cohort of patients with VS who were treated at our centre using a linear accelerator-based stereotactic radiosurgery system.
METHODS
We retrospectively reviewed the case notes and magnetic resonance (MR) images of patients with VS treated with radiosurgery. Treatment was administered as either a single 13 Gy session or 25 Gy in five sessions. At our centre, only larger or higher Koos grade VSs, were routinely treated with hypofractionated radiosurgery. Tumour response and hearing were assessed using RECIST criteria and Gardner-Robertson scale, respectively. Other toxicities were assessed using physical examination and history-taking. Freedom from radiological progression was estimated with the Kaplan-Meier method.
RESULTS
46 patients received single-fraction radiosurgery and 31 received hypofractionated radiosurgery. Median follow-up duration was 40.6 months. 29 patients had prior surgery to remove the tumour (median size 1.68 cm3). One patient who had symptomatic increase in tumour size (> 20% in largest diameter) was treated conservatively and subsequently showed stable disease on MR imaging. Progression-free survival was 98.7%. Another patient had symptomatic oedema requiring ventriculoperitoneal shunt insertion. 11 patients had serviceable hearing before radiotherapy and 72.7% of them retained useful hearing (20.1 dB mean decline in pure tone average). Facial and trigeminal nerve functions and sense of equilibrium were preserved in > 90% of patients.
CONCLUSION
Radiosurgery is effective and safe for small VSs or as an adjunct therapy after microsurgery.
INTRODUCTION
Vestibular schwannomas (VSs) are benign tumours of the eighth cranial nerve. Treatment options include microsurgery, fractionated stereotactic radiotherapy (FSRT), stereotactic radiosurgery (RS), and a combination of surgery and radiotherapy (RT) or observation. Decision on treatment is made based on specific factors, such as the size of the VS, presenting symptoms, the patient’s age, performance status and comorbidities, and whether the goal is hearing preservation. A recent Cochrane analysis, which aimed to look for randomised evidence in the treatment of VSs, concluded that therapy should be tailored to the individual, as the study could not find any high-quality evidence from randomised controlled trials.(1) In a prospective study, Breivik et al showed that RS reduces tumour growth and extends treatment rate by tenfold, as compared with conservative management; they also found similar hearing preservation rates in both arms.(2) To date, there are no randomised comparisons of RS versus surgery. Another systematic review, which analysed six controlled studies comparing RS and microsurgery, concluded that RS showed better outcomes for VS (up to 30 mm in cisternal diameter).(3) Stereotactic RS comprises linear accelerator (LINAC)-based or gamma knife RS and FSRT. The Stereotactic Radiosurgery Task Force defines RS as high-dose hypofractionated RT performed for up to a maximum of five sessions.(4) In theory, RS is more effective for local control, as it supposedly has a low a-b ratio for VS; however, it is also believed that fractionated RT has a better toxicity profile from a radiobiological viewpoint. No large randomised trial has addressed the differences in outcomes between RS and FSRT, although one large study of 449 patients, which pooled the results from three German centres, did show that both treatments were equally effective if the patients were chosen diligently based on tumour volume (the patients in the RS group had smaller tumours), pre-treatment characteristics and RS prescription ≤ 13 Gy.(5) To combine both the efficacy of single-fraction RS and the fractionated benefits of FSRT, hypofractionated treatment in 3–5 fractions has been proposed. This study was conducted to contribute our centre’s experience with a LINAC-based RS regimen (both single-session and hypofractionated).
METHODS
Between 2007 and 2014, 81 VS patients were treated with RS at the Department of Radiation Oncology, National Cancer Centre, Singapore. Only patients with follow-up data were included in the study. In total, we analysed 77 patients. The study was approved by the SingHealth Centralised Institutional Review Board.
In our institution, only smaller tumours or patients who are not keen on surgery are treated with RS. Patients with good, serviceable hearing are offered FSRT instead of RS in the belief that FSRT may be better for hearing preservation. Before treatment, all patients were seen by a multidisciplinary team that consisted of neurosurgeons, ear, nose and throat surgeons, and radiation oncologists (with radiologist-reported magnetic resonance [MR] imaging). Treatment decisions were made based on multidisciplinary consensus. Generally, patients were treated only if they were symptomatic (i.e. none of the 77 patients were ‘incidentalomas’), and factors like comorbidities, life span and tumour growth rate were given consideration prior to treatment. Following resection, patients were treated only if the tumours showed progression. Standard T1- and T2-weighted gadolinium-enhanced MR imaging with 1-mm slice thickness was carried out. For the purpose of immobilisation, a customised thermoplastic head frame was made for every patient.
The Brainlab™ treatment planning system (Brainlab AG, Munich, Germany) was used and patients were treated with the Novalis™ Shaped Beam Radiosurgery System (Brainlab AG), which was equipped with 3-mm micro-multileaf collimators and the ExacTrac image-guidance system (Brainlab AG). Fine-cut computed tomography simulation images were registered with recently acquired contrast-enhanced MR imaging and CISS (constructive interference in steady state) sequence MR images for target delineation. Both the radiation oncologists and neurosurgeons were involved in tumour contouring. A planning target volume (PTV) margin of 1–2 mm was given to account for intra-fraction motion and setup uncertainties. A marginal dose of either 12–13 Gy or 25 Gy in five fractions was prescribed to the PTV. An average of 7–9 conformal beams were used to create the treatment plan using a single isocentre. In cases where the lesion was abutting the brainstem or cochlear, intensity-modulated radiosurgery was used to achieve the dose constraints required. The majority of cases were normalised to the 80% isodose line, and the median conformality index in our cohort was 1.29 (maximum allowable < 2). The minimum dose received by the PTV was 95% of the prescription dose, and no hotspots exceeding 100% of the prescription dose were allowed outside the PTV. Dose constraints were a maximum dose of 12 Gy to the brainstem and < 5 Gy to the ipsilateral cochlear if hearing preservation was a priority. For patients receiving 5 × 5 Gy fractions (hypofractionated RS), the brainstem constraint was V23 < 0.5 cm3 and the maximum permissible dose limit was 30 Gy in five fractions. Image-guided RT tolerance was set to 0.7 mm and 1° using the ExacTrac image-guidance system, and the patient was repositioned using the 6D robotic couch to correct for positioning errors, with final confirmatory ExacTrac verification before treatment was commenced. This process was repeated with every change in gantry angle to ensure accurate delivery.
Fig. 1
Dose colour-wash images show the treatment plan for a Koos Grade 4 tumour.
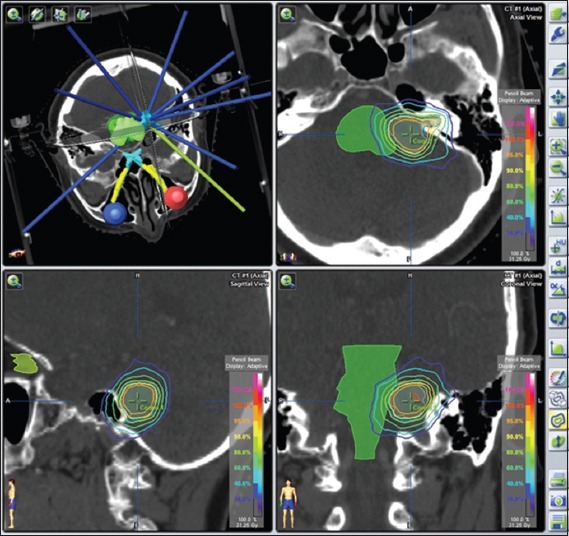
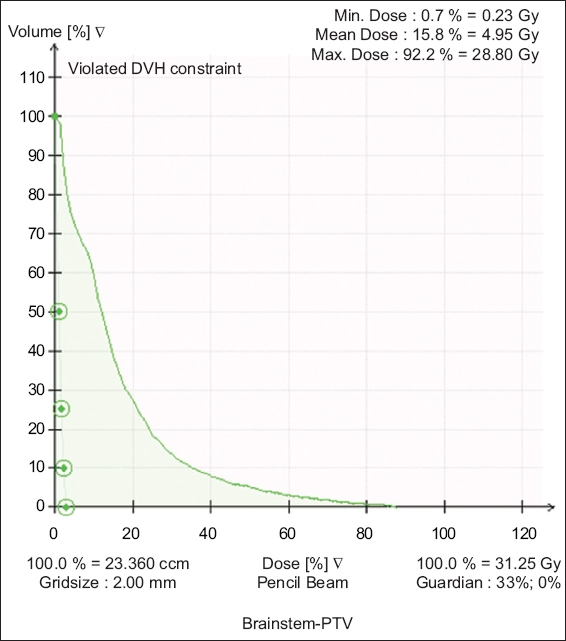
Fig. 2
Graph shows the dose-volume histogram for a Koos Stage 4 tumour treated with 25 Gy in five fractions. DVH: dose-volume histogram; PTV: planning target volume
Statistical analysis was performed with IBM SPSS Statistics version 19.0 (IBM Corp, Armonk, NY, USA). Freedom from progression and surgery outcomes were assessed using the Kaplan-Meier method.
RESULTS
A total of 77 patients were treated with RS.
Table I
Pre-treatment characteristics of patients (n = 77).
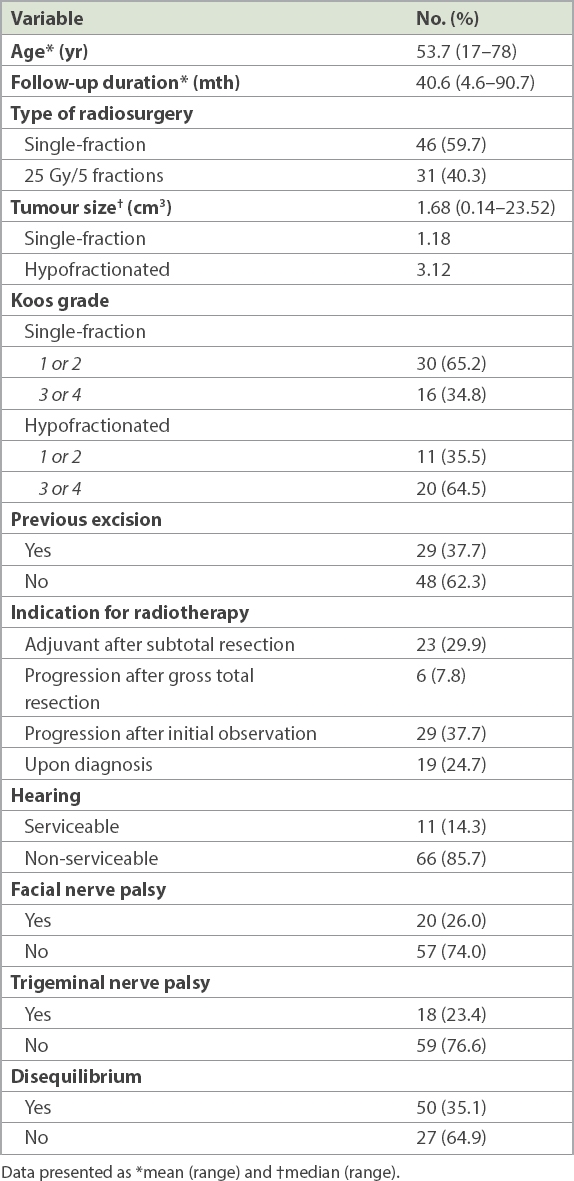
In all, 36 patients were in Koos Grade 3 or 4 (i.e. the tumour was in contact with or compressing the brainstem). Out of the 46 patients who received single-fraction treatment, 16 (34.8%) were Koos Grade 3 or 4. Among the 31 who received hypofractionated treatment, 20 (64.5%) were Koos Grade 3 or 4. 29 patients had prior surgery – 23 received radiation after subtotal resection, and six had tumour progression despite gross total resection. 48 patients did not have prior resection of tumour – 29 received RT due to progression despite an initial period of observation and 19 received RT upon diagnosis. None of the 77 cases were ‘incidentalomas’ who had exhibited symptoms that prompted further investigations and subsequent treatment. Only 11 of the 77 patients had pre-RT serviceable hearing (i.e. PTA ≤ 50 dB or able to use the telephone with the affected ear). Prior to RS, 20 patients had facial nerve palsy, 18 had trigeminal nerve palsy and 50 had disequilibrium. Of the 20 with facial nerve palsy, all had undergone prior resection.
Following RS, none of the 46 patients who received single-fraction treatment had progression of tumour or required surgery. Of the 31 who received hypofractionated treatment, one had radiological progression (
Table II
Post radiosurgery response on magnetic resonance (MR) imaging (n = 77).
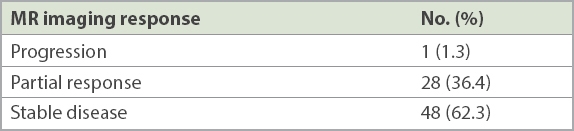
Fig. 3
Graph shows the proportion of progression-free patients.
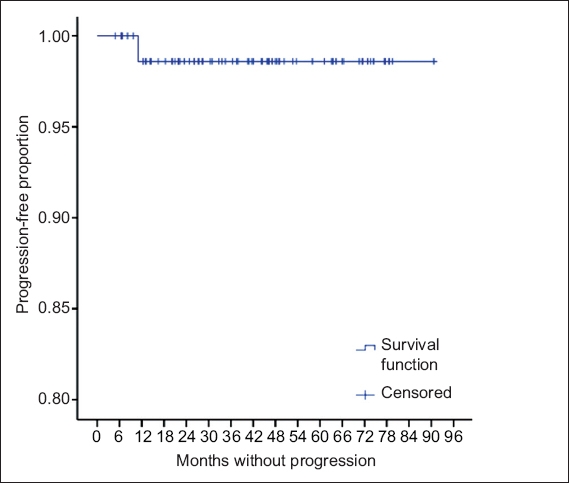
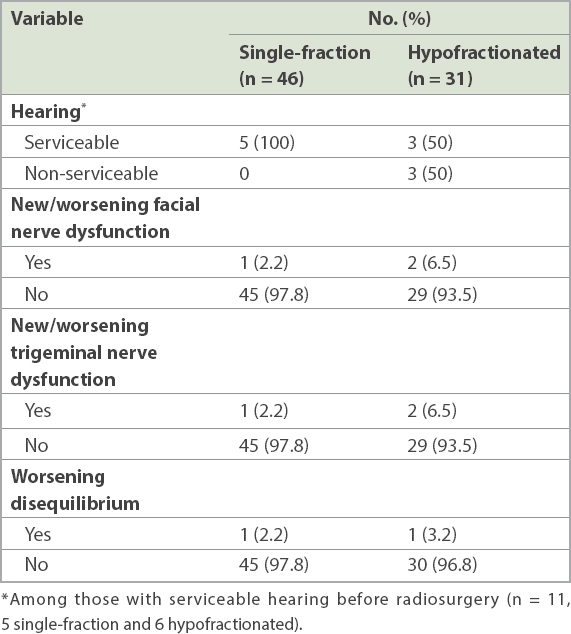
The majority of patients retained good cranial nerve function after RS (
Table III
Post radiosurgery cranial nerve function of patients who had single fraction vs. hypofractionated radiosurgery.
DISCUSSION
In our institution, only patients with smaller tumours are offered RS. Larger tumours receive either FSRT, or surgery upfront with or without adjuvant RS. For Koos Grade 3 or 4 tumours, we tend to offer hypofractionated instead of single-fraction RS. This study has shown that with careful patient selection – especially with regard to tumour size – patient outcomes were excellent, with freedom from progression and freedom from surgery both at 98.7%. Many other larger studies have also confirmed the safety of single-fraction RS,(5,7-10) although far fewer and smaller studies have reported on hypofractionated RS.(11-14)
Our patient who had peritumoral oedema requiring VP shunt insertion had a larger tumour (7.17 cm3, median 1.68 cm3) prior to RS, and this could have been a predisposing factor for complications. Chan et al’s study(9) on FSRT found that tumour size was strongly predictive of neurosurgical complications, and that three- and five-year actuarial rates of freedom from any neurosurgical intervention were 100% and 97% for patients with tumour volume < 8 cm3, but were 74% and 47% for tumour volume > 8 cm3. The authors also recommended that large tumours be debulked maximally while preserving cranial nerves and that adjuvant RS should be given for subtotal resection or for tumour regrowth following gross total resection.(9) This approach has been supported by Iwai et al,(15) who published a paper on a series of 40 patients with large tumours (median maximum diameter 32.5 mm). In this study, the authors reported that planned partial surgical resections followed by gamma knife RS had a good tumour control rate of 86% and a high rate of facial nerve and hearing preservation. However, in another study comprising a cyberknife series of 33 VSs with tumour volume > 8 cm3 treated with 2–5 fractions (14.0–19.5 Gy), only two patients were reported to require debulking, with another two requiring shunts.(16) Another study by Aoyama et al(17) showed that size was predictive of tumour progression. Their series, which included 201 patients who were treated with FSRT, reported tumour expansion rates of 0%, 11.4%, 25.6% and 50% in tumours measuring 9 mm, 10–19 mm, 20–29 mm and 30 mm, respectively; in all, only 5% of patients required surgical salvage.(17) In our study, although the patient with radiological progression had a small tumour (1.55 cm3), there was a transient increase in size with resultant facial nerve compression, probably due to treatment reaction. This patient was managed conservatively without further symptoms or tumour progression. The phenomenon of transient increase in tumour size has been reported previously; a Swedish study from Karolinska Institutet, comprising a series of 254 patients, reported that 12% of VSs continued to enlarge temporarily over 6–18 months after treatment and subsequently either shrank or stabilised.(18) However, it is not clear what the definition of size increase was in this Swedish study. Another large series of 496 patients from John Hopkins Hospital,(19) in which the majority were treated with 25 Gy over five fractions, reported that 30% of patients experienced radiologic progression. In the John Hopkins study, radiologic progression was defined as “tumour volume greater than the baseline volume and a stable clinical status at follow-up”(19) and the method for assessing tumour volume also differed from that used in the current study. In our study, only the presence of tumours that fulfilled RECIST criteria 1.1 for progression were defined as progression. All in all, we recommend that early mild progression be monitored with serial MR imaging instead of immediate resection.
In our series, 36 out of 77 patients had Koos Grade 3 or 4 tumours. Koos Grade 4 tumour is defined as a tumour with extension to the external auditory canal > 30 mm or causing brainstem compression. This definition is not absolutely clear, as brainstem compression runs a spectrum from mild indentation of the brainstem to gross contortion of the brainstem and fourth ventricle.(20) Although the clinical outcome was excellent in our current study, it has also been reported that brainstem compression is a risk factor for progression. Hasegawa et al’s study of 440 gamma knife surgical patients found that brainstem compression with fourth ventricle compression significantly resulted in worse outcome.(10) Another smaller report of 65 patients with larger-volume VS by Yang et al(21) similarly found that Koos Grade 4 tumours were less likely to have tumour control; in that study, nine out of the 65 patients had larger tumour volumes requiring intervention. Their median tumour volume of 9 cm3, which is much larger than that of our series, could explain the higher rate of complications.(21) In our institution, gross brainstem or fourth ventricular distortion with symptomatic mass effect is a relative contraindication for RS.
Only 11 patients in our study had pre-RS serviceable hearing. This is because our institution tends to offer FSRT to patients who have good hearing prior to RT. Among these 11 patients, the rate of hearing preservation was 72.7% and the decline in mean PTA was 20.1 dB. A Japanese series also reported a similar mean PTA decline of 27.3 dB.(14) An analysis of 4,234 patients from multiple institutions treated with gamma knife RS showed an overall hearing preservation rate of about 51% 3–4 years after treatment.(22) In our study, it is interesting to note that the rate of hearing preservation was much lower (50%) in the hypofractionated treatment group. Similar to our findings, a report of 29 patients treated with 7 × 4 Gy reported a five-year actuarial Class A/B (pure-tone thresholds ≤ 50 dB and speech discrimination ≤ 50%) hearing preservation rate of 50%.(12) Another report of 27 patients who underwent hypo-FSRT found that none of the patients treated with 20–24 Gy in 5–6 fractions preserved serviceable hearing.(23) However, another report of patients treated with cyberknife in 2–5 fractions (14–19.5 Gy) showed that seven out of eight patients with baseline serviceable hearing retained their hearing,(16) although the doses used were lower compared with other studies. Hypofractionated RS needs to be further evaluated for hearing preservation safety.
Our rates of cranial neuropathies were low, with more than 90% of patients having no worsening or new facial and trigeminal nerve palsies, or worsening disequilibrium. This is comparable to other studies, as outlined in Murphy and Suh’s review of FSRT and RS, which reported a cranial nerve V and VII preservation rate of more than 95%.(24)
It has been reported that the general risk of hearing deterioration after surgery is 30%–50% and that of local control is 80%–98%, and the risk of mortality has been cited as less than 2% in most series.(25,26) For patients who are not surgical or general anaesthetic candidates, RT is a good option. Even for fit patients, some opine that RS is the treatment of choice for smaller uncomplicated tumours. A systematic review of only controlled studies that compared microsurgery, RS and fractionated RT concluded that for solitary VS up to 30 mm in cisternal diameter, RS showed better outcomes compared to microsurgery, as there were no direct mortality and surgical or anaesthetic complications, and patients had better preservation of facial nerve and hearing, as well as better quality of life.(3) However, the review could not find any randomised or controlled studies on fractionated RT. Although the systematic review included six studies comparing RS and microsurgery, only four studies(27-30) were deemed to be of good quality with no relevant bias identified. In these four studies, facial nerve was intact in 67%–82% and 91%–100% of microsurgical and RS patients, respectively, while hearing preservation was 0%–36% and 32%–75%, respectively. Hence, we suggest that small, uncomplicated tumours be treated with RS. However, for patients who have tumours with hydrocephalus, surgery is the preferred option, as they will benefit from the immediate relief of pressure.
In choosing between FSRT and RS, there are a few factors to take into consideration. Some clinicians believe that due to the radiobiological benefit of a smaller dose per fraction, FSRT has the benefits of hearing preservation and increased safety for larger tumours. As reported in a series of 99 patients treated at Addenbrookes Hospital, United Kingdom, with 50 Gy in 30 fractions conformal RT, the actuarial local control rate was 96.9%, hearing preservation 100%, facial nerve preservation 96.8% and trigeminal nerve preservation 100%.(31) In a review published in 2014, Jian et al concluded that fractionated RT is an excellent treatment alternative to RS, especially in patients with larger tumours, largely due to its excellent tumour control and toxicity profile.(32) On the other hand, FSRT is logistically more inconvenient, as it takes up more of the patient’s time and may also add strain to busy treatment units. Unfortunately, there is no direct study comparing FSRT and RS in the literature. Most studies are retrospective, and subjects in the RS arm had smaller and less complicated tumours. In a study of 104 patients treated with RS, conventional FSRT (45–50.4 Gy in 35–28 fractions) or hypo-FSRT (20 Gy in 5 fractions), the five-year progression-free and cranial nerve toxicity rates were equivalent. However, there was no comparison of tumour size, and the patients treated with FSRT had better baseline hearing as well as facial and trigeminal nerve functions.(33) Studies looking at outcomes and toxicities of FSRT reported good local control, good hearing and cranial nerve preservation, with hearing preservation of 73%–98%,(7,9,31,34-37) as well as better hearing preservation at the one-year follow-up compared to RS.(7) With a longer follow-up of 67 months, a pooled German study found no difference in loss of useful hearing rates between the FSRT and stereotactic RS groups (14% vs. 16%).(5) However, it is admittedly difficult to compare the rate of hearing preservation among different studies due to the different recording methods used (such as the Gardner-Robertson scale, word discrimination method and subjective reporting). Although RS is not recommended for large VSs, there was a single case report of a large VS (3.8 cm in diameter) treated with FSRT following VP shunt placement that showed good tumour control and no cranial nerve or hearing morbidity at 63 months of follow-up; the authors thus concluded that FSRT was a potential noninvasive treatment modality for larger tumours.(38)
To combine the efficacy of single-fraction RS and the supposed safety of FSRT, some clinicians have proposed treatment with hypofractionated RS. In the present study, patients who received hypofractionated RS had larger tumours and tended to be in Koos Grade 3 or 4, as it is believed that hypofractionated RS may confer more clinical safety for riskier tumours. A Japanese series of 25 patients with 26 VSs treated with hypofractionated RS showed a seven-year progression-free rate of 95%. Of the 12 patients who had PTA < 50 dB before RS, the mean PTA before and after hypofractionated RS was 29.8 dB and 57.1 dB, respectively. 2 (7.7%) patients developed Grade 2 facial nerve disorder.(14) In a German series of 29 subjects treated with 7 × 4 Gy, the five-year hearing preservation rate was 50%. It also reported a transient increase in tumour volume in 17 out of 29 patients; however, it was unclear how tumour volume increase was defined. After a median follow-up period of 89.5 months, only one patient showed radiological progression and no patient required intervention.(12) Another study on benign skull base tumours, which included ten VSs treated with 5 × 5 Gy, local control and cranial nerve function preservation were reported in all the patients.(13) Other series on hypofractionated RS have reported similar outcomes, including a Japanese study(23) and Taiwanese(11) study, both of which reported no permanent facial or trigeminal nerve morbidity and no salvage surgery. Another study reported only one case each of vertigo, tongue paraesthesia and trigeminal neuralgia among the 33 cases of large VSs.(16) With these studies in mind, the safety profile (excluding hearing preservation) of hypofractionated RS appears to be quite established, although experience with hypofractionated RS has been reported much less frequently than single-fraction RS.
Malignant transformation of a tumour or secondary neoplasm from irradiation exists, although the risk is small. Balasubramaniam et al’s(39) review of secondary tumours after stereotactic RT found that only 20 cases have been reported as of 2007. Of the 20 patients, 14 had VS. Eight of the VS patients had NF-2, six of whom had pathological confirmation of malignant transformation 2–6 years after RT when they presented with accelerated tumour growth. The histology was malignant nerve sheath tumour in most of the cases.(39) In another report of 440 patients who were followed up for more than ten years after gamma knife surgery for VS, only one developed malignant transformation.(10) Therefore, although the risk of secondary tumours is small, this information must be conveyed to patients, especially younger or NF-2 patients, prior to RT.
Another rare but serious complication of RT is brainstem radiation necrosis. A study of 93 VS patients treated with FSRT reported a single case of probable radionecrosis of the brainstem with serious complications that manifested nine months after FSRT. This patient was found to have been treated within brainstem tolerance and thus the authors postulated that the patient’s previous surgical excision of the tumour could have reduced his brainstem tolerance.(36)
Bearing the pros and cons of the various treatment options in mind, observation may sometimes be a valid and the most reasonable option for some patients, given the indolent natural history of VS, especially in patients with a limited estimated lifespan or poorer performance status, or those who are asymptomatic or incidentally diagnosed during brain MR imaging. In the current series, 19 patients had RT upon diagnosis of VS. Although all 19 were symptomatic, their tumours may not have progressed during the follow-up period.
Going forward, prospective trials comparing the different treatment modalities are required, although with high tumour control and low toxicity rates across all treatment options, obtaining statistically significant results may be difficult, as a large number of patients will be needed. To better compare between studies, standardising the reporting of tumour size (cm3 or maximal diameter) and tumour control, such as RECIST criteria and toxicity reporting, should be undertaken for future trials or cohort reporting.
The majority of the VS patients in the current study were treated only after a period of initial observation, having shown evidence of size progression. Hughes et al(40) reported that in a retrospective series of 59 patients who were chosen for initial observation, only 11 (19%) eventually required intervention. They also found that tumours that had already extended into the cerebellopontine angle had a significantly faster rate of growth than intracanalicular tumours.(40)
The present study was not without limitations. Firstly, our hearing preservation results were subject to bias, as only 11 of our patients had serviceable hearing before treatment (our institution’s practice is to offer FSRT only to patients with good baseline hearing). Secondly, toxicity scoring, facial nerve palsy, post-RS disequilibrium and trigeminal neuropathy were based on subjective reporting of symptoms and not objectively measured with clinical tests or scores such as electronystagmography or House-Brackmann score. As grading of facial nerve function was not rigorously done, the low rate of facial nerve palsy after RS may not be accurate. Finally, the decision to treat with single-fraction or hypofractionated RS was not made based on definite or clear-cut departmental guidelines, except that larger tumours with more brainstem mass effect were more likely to be treated with a hypofractionated regimen.
In conclusion, small, uncomplicated but enlarging VSs should be treated with RS, although observation is an option for a selected subgroup, especially older patients or those with small, asymptomatic and slow-growing tumours. Observation can also be practised after subtotal resection, as shown in a report in which only 18 out of 116 patients had recurrence after subtotal resection.(41) RS is efficacious with good safety records. Microsurgery can be reserved for larger, more complicated VSs, with adjuvant RS an option for recurrences. Between FSRT and RS, it is unclear if the former offers better hearing preservation in the long run.
ACKNOWLEDGEMENTS
We would like to thank Dr Chua Eu Tiong and his team of radiation oncologists from the Department of Radiation Oncology, National Cancer Centre Singapore, and SingHealth neurosurgeons and physicists who were involved in the care of the patients in this study.


