Abstract
INTRODUCTION
Acute myocardial infarction (AMI) due to unprotected left main coronary artery (ULMCA) disease is clinically catastrophic although it has a low incidence. Studies on the long-term prognosis of these patients are rare.
METHODS
From January 1999 to September 2013, 55 patients whose infarct-related artery was the ULMCA were enrolled. Clinical, angiographic and interventional data was collected. Short-term and long-term clinical follow-up results as well as prognostic determinants during hospitalisation and follow-up were analysed.
RESULTS
Cardiogenic shock (CS) occurred in 30 (54.5%) patients. During hospitalisation, 22 (40.0%) patients died. Multivariate logistic regression analysis showed that CS (odds ratio [OR] 5.86; p = 0.03), collateral circulation of Grade 2 or 3 (OR 0.14; p = 0.02) and final flow of thrombolysis in myocardial infarction (TIMI) Grade 3 (OR 0.05; p = 0.03) correlated with death during hospitalisation. 33 patients survived to discharge; another seven patients died during the follow-up period of 44.6 ± 31.3 (median 60, range 0.67–117.00) months. The overall mortality rate was 52.7% (n = 29). Kaplan-Meier analysis showed that the total cumulative survival rate was 30.7%. Cox multivariate regression analysis showed that CS during hospitalisation was the only predictor of overall mortality (hazard ratio 4.07, 95% confidence interval 1.40–11.83; p = 0.01).
CONCLUSION
AMI caused by ULMCA lesions is complicated by high incidence of CS and mortality. CS, poor collateral blood flow and failure to restore final flow of TIMI Grade 3 correlated with death during hospitalisation. CS is the only predictor of long-term overall mortality.
INTRODUCTION
Acute myocardial infarction (AMI) due to unprotected left main coronary artery (ULMCA) disease is clinically catastrophic although it has a low incidence. Due to the high likelihood of cardiogenic shock (CS) as a complication, patients with AMI due to ULMCA disease have a high mortality rate even after successful revascularisation.(1-5) Studies on the prognosis of these patients, especially in the long term, are rare. In addition, follow-up durations reported in previous published research were short and therefore cannot show the long-term prognosis of these patients.(4-6) In the present study, we analysed the short- and long-term prognoses of patients with AMI due to ULMCA disease as well as the predictors of these prognoses. Only AMI patients whose infarct-related artery (IRA) was the ULMCA, as confirmed by angiography, were enrolled and long-term follow-up was performed.
METHODS
From January 1999 to September 2013, 5,578 patients in Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, received emergency coronary angiography; patients whose IRA was confirmed to be the ULMCA were enrolled in this study. Clinical, angiographic and interventional characteristics as well as short- and long-term clinical follow-up results were analysed.
The diagnosis of AMI requires detection of a rise and fall of cardiac biomarker values (e.g. cardiac troponin), symptoms of ischaemia and changes on electrocardiography.(7) Coronary flow in the IRA before and after revascularisation was graded according to the classification system of the thrombolysis in myocardial infarction (TIMI) study group.(8) Collateral flow was graded using a classification system developed by Rentrop et al.(9) ULMCA disease was defined as a lesion ≥ 50% in the left main artery in the absence of a patent coronary artery bypass graft (CABG) to the left anterior descending (LAD) or circumflex arteries.(10,11) CS was defined as hypotension secondary to cardiac dysfunction (i.e. systolic blood pressure of < 90 mmHg for at least 30 minutes or the need for supportive measures to maintain a systolic blood pressure of ≥ 90 mmHg) and end-organ hypoperfusion.(12) The endpoint of the study was set as all-cause mortality. Patients and their families were followed-up via telephone interviews, and outpatient and inpatient medical records were collected. Patients were divided into two groups depending on whether death occurred during hospitalisation or follow-up; predictors of death during hospitalisation and long-term follow-up were analysed.
Data analysis was performed using SPSS Statistics version 17.0 (SPSS Inc, Chicago, IL, USA). Continuous data was expressed as mean ± standard deviation, while discrete data was expressed as percentages. Student’s t-test and chi-square test (Fisher’s exact test when necessary) were used to analyse continuous and discrete data, respectively. Logistic regression analysis was used to analyse factors associated with death during hospitalisation. Cox regression analysis was performed to analyse the risk factors of death during follow-up; univariate analysis was conducted and statistically significant factors were then included in multivariate analysis. Kaplan-Meier analysis was used to estimate the cumulative survival rate. Statistical significance was set at p < 0.05 in a two-sided test.
RESULTS
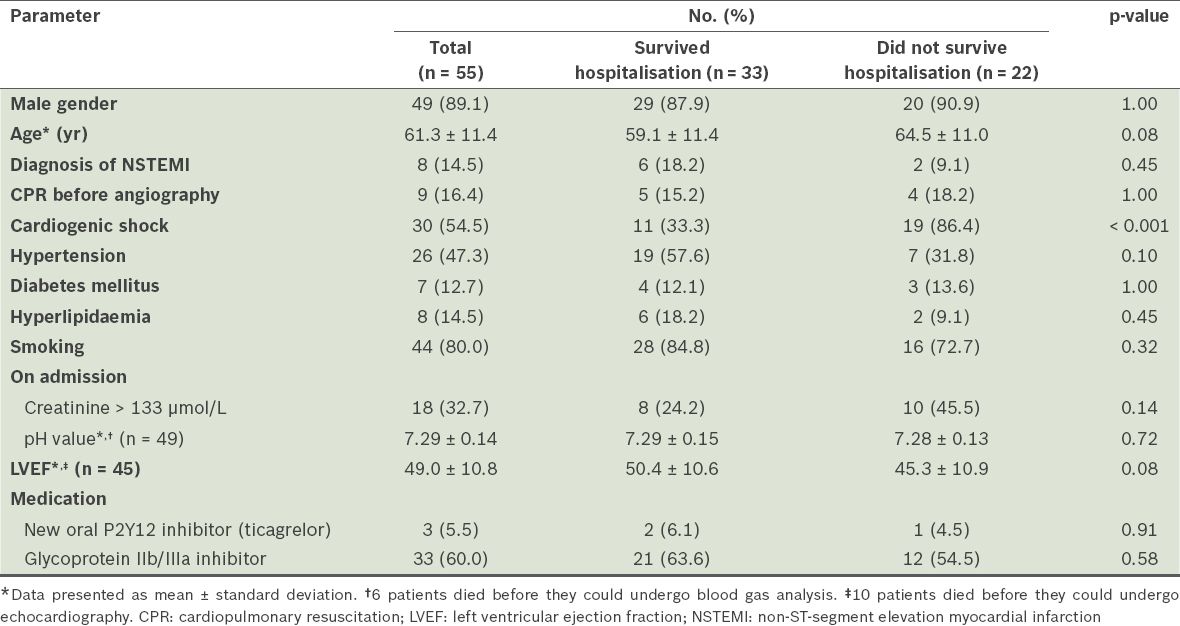
A total of 55 patients were included in the study. Their mean age was 61.3 ± 11.4 (range 41–86) years and 49 patients were male. 47 (85.5%) patients had an acute, extensive, anterior wall ST-segment elevation myocardial infarction (STEMI) and the remaining 8 (14.5%) had acute non-ST-segment elevation myocardial infarction (NSTEMI). There were five cases of stent thrombosis in the left main coronary artery (LMCA), including two cases of subacute stent thrombosis, two cases of late stent thrombosis and one case of very late stent thrombosis. Of the two patients with subacute stent thrombosis, one had a history of CABG with occluded bypass graft. CS occurred in 30 (54.5%) patients. Among the remaining 25 patients, there were ten cases of Killip class III, eight cases of Killip class II and seven cases of Killip class I. Echocardiography was not performed in ten patients before they died; in the other 45 patients, postoperative echocardiography showed a mean left ventricular ejection fraction (LVEF) of 49.0% ± 10.8% (range 24.0%–70.0%) (
Table I
Baseline clinical characteristics of the patients.
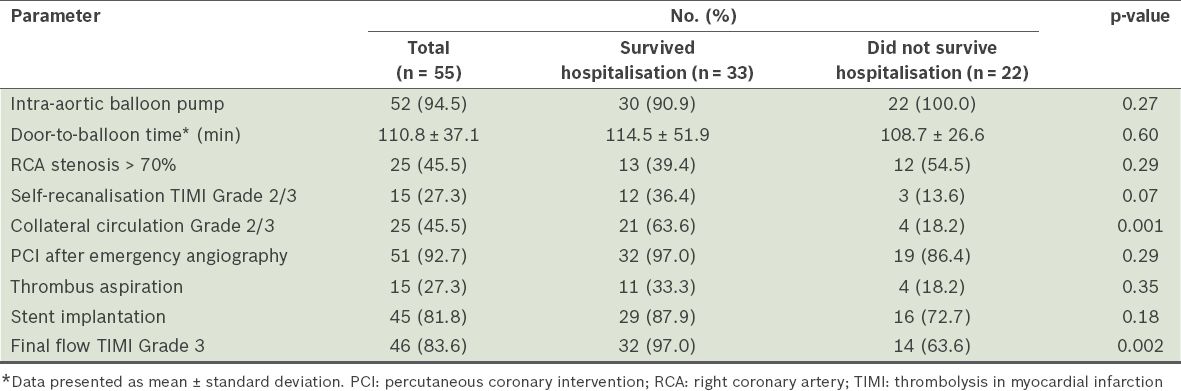
All patients had a dominant right coronary artery. 51 patients underwent emergency percutaneous coronary intervention (PCI), with a mean door-to-balloon time of 110.8 ± 37.1 (range 57–300) minutes. Of the 51 patients, 45 received stent implantation and six received only balloon angioplasty, including three cases of elective CABG after emergency PCI. In the patients who received stent implantation, the stent covered the LAD and LMCA in 39 cases. In two cases, the attempt to open the LAD failed, and the stent covered the circumflex artery and LMCA; in the remaining four cases, double stent procedure was performed for bifurcation lesions. Emergency CABG was performed directly in one patient; in another, self-recanalisation occurred and elective CABG was performed two weeks after emergency angiography. Another two patients died soon after receiving emergency coronary angiography and revascularisation could not occur. 52 patients (94.5%) received intra-aortic balloon pump (IABP) implantation (
Table II
Angiographic and interventional data of the patients.
A total of 22 (40.0%) patients died in hospital: five patients died during angiography or PCI and the remaining 17 died within 3.0 ± 4.5 (range 1–21) days after onset of AMI. In all cases, the primary cause of death was refractory CS. One patient suffered from subacute stent thrombosis 11 days after primary PCI and died two days after the second PCI, which was balloon angioplasty. Multivariate logistic regression analysis revealed that the incidence of CS during hospitalisation (odds ratio [OR] 5.86), collateral circulation of Grade 2 or 3 (OR 0.14) and final flow of TIMI Grade 3 (OR 0.05) correlated with mortality during hospitalisation (
Table III
Predictors of mortality during hospitalisation.
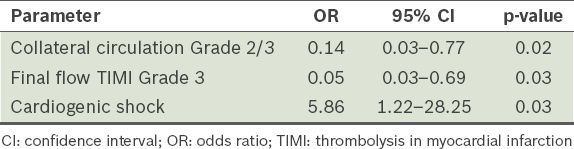
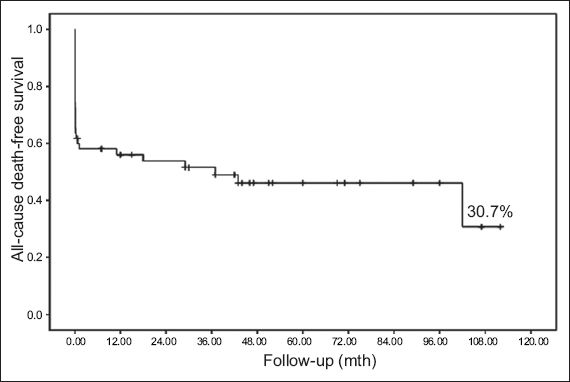
Fig. 1
Kaplan-Meier survival curve shows cumulative survival rates for all patients.
Univariate Cox regression analysis revealed that age ≥ 60 years, presence of CS, collateral circulation of Grade 2 or 3, and final flow of TIMI Grade 3 correlated with overall mortality. Multivariate analysis showed that CS was the only independent predictor of overall mortality (hazard ratio 4.07, 95% confidence interval [CI] 1.40–11.83; p = 0.01) (
Table IV
Cox regression analysis of the predictors of total mortality.
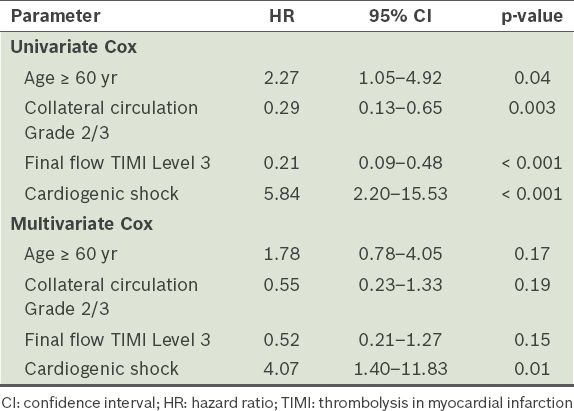
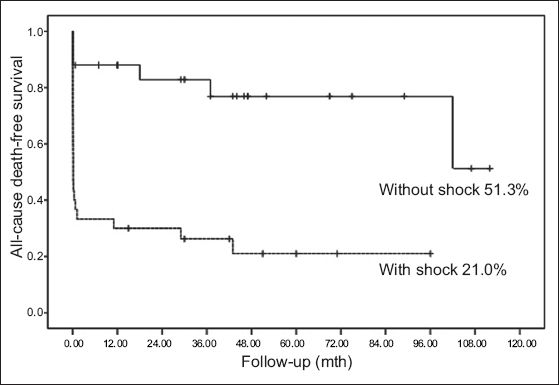
Fig. 2
Kaplan-Meier survival curve shows cumulative survival rates of patients with and without cardiogenic shock.
DISCUSSION
AMI due to ULMCA disease is catastrophic; however, not all patients are able to undergo emergency coronary angiography for confirmation. For this reason, its true incidence may be unclear. In a study by Izumikawa et al, of 3,212 patients who received emergency coronary angiography, the IRA was confirmed to be the ULMCA in 72 (2.2%) cases.(2)
Among AMI patients receiving emergency PCI, the ULMCA is the culprit lesion in 1.1%–2.2%.(4,5,13) There is a high incidence of CS (46%–78%) in these patients.(1-5) Even after receiving successful reperfusion therapy, their acute-phase mortality was still as high at 39.7%–55%.(1-5) Previous studies focused mostly on predictors of acute-phase prognosis. A univariate analysis by Lee et al showed that self-recanalisation of the LMCA (TIMI Grade 2 or 3) was the determinant prognostic factor,(3) while Iwasaki et al proposed that a dominant right coronary artery and well-developed collateral circulation might be the prognostic factor.(14) In the present study, all patients had a dominant right coronary artery; the patients with small right coronary arteries might have had little opportunity to receive emergency coronary angiography. In a study by Hurtado et al, multivariate analysis showed that CS and incomplete revascularisation correlated with in-hospital mortality.(15) In another study by Parma et al, multivariate analysis showed that CS, age ≥ 75 years and postoperative blood flow of TIMI Grade 3 correlated with the 30-day mortality rate.(5) Similarly, our analysis suggests that CS (OR 5.86), well-developed collateral circulation (OR 0.14) and final flow of TIMI Grade 3 (OR 0.05) correlated with death during hospitalisation.
Few studies of patients with AMI due to ULMCA disease involved long-term follow-up. Sakai et al reported that the one-year mortality rate of shock patients was significantly higher than that of patients without shock.(4) In the study by Parma et al, shock, age ≥ 75 years old, and final flow correlated with mid-term follow-up results; Kaplan-Meier analysis showed the overall mid-term survival rate to be approximately 55.9%, while the overall mid-term survival rate of patients with CS was 33.8%.(5) In Izumikawa et al’s study of the long-term prognosis of LMCA occlusion patients after emergency PCI, Kaplan-Meier analysis showed the total cumulative survival rate during follow-up to be 26.2% and that of shock patients to be 21.1%.(2) The results of the current study were similar: the overall mortality rate was 52.7% (n = 29), while the total cumulative survival rate for all patients was 30.7% and that of patients with CS was only 21.0%, suggesting a poor long-term prognosis for patients with CS.
In these prior studies, the Kaplan-Meier survival curves tended to be flat during mid- or long-term follow-up.(2,5) Both Izumikawa et al and Parma et al proposed that the mid- and long-term prognosis of surviving patients might be relatively good.(2,5) Similarly, Gagnor et al also reported that the rate of adverse events in patients after discharge was low.(6) However, the Kaplan-Meier survival curve did not show similar results in the present study. This may be related to differences in follow-up time. In the study by Parma et al, follow-up time was 15.8 ± 10.9 months, with a median of only 14 months.(5) The follow-up times of the studies carried out by Izumikawa et al and Gagnor at al were also only 1.7 ± 2.9 years and 504 ± 653 days, respectively,(2,6) which was substantially shorter than the follow-up time in the current study, which was 44.6 ± 31.3 (median 60) months.
Previous studies have also shown that acute LMCA occlusion may lead to ST-segment elevation in the anterior wall as well as widespread ST-segment depression. Marso et al reported that, among 34 cases, there were 24 cases of ST-segment elevation.(1) In Hurtado et al’s study, STEMI only accounted for 59% of cases.(15) However, in studies by Lee et al and Parma et al, all enrolled patients had STEMI.(3,5) As Iwasaki et al indicated, variations in ECG manifestation are related to differences in coronary artery dominance and collateral circulation.(14) In addition, they may also be related to differences in indications for emergency angiography in each study. Kim et al reported in their study that the proportion of STEMI among patients with CS was higher than in patients without CS.(16) In our study, however, NSTEMI accounted for 14.5% of all cases and the rate of occurrence did not differ between the groups that survived and did not survive hospitalisation.
The optimal revascularisation strategy should be decided by a heart team that includes an interventional cardiologist and a cardiac surgeon. For the majority of patients with AMI due to ULMCA disease who suffer from severe CS, Iwasaki et al indicated that thrombolytic therapy could not produce satisfactory reperfusion and that emergency PCI should be the preferred treatment;(14) if PCI fails, emergency CABG may be considered. In a study comparing outcomes after percutaneous or surgical revascularisation of ULMCA-related AMI by Grundeken et al, the 30-day mortality rates of the PCI and CABG groups were 64% and 24%, respectively.(17) However, in Grundeken et al’s study, there was an obvious bias regarding the selection of revascularisation strategy: for patients with TIMI Grade 0 or 1 in angiography, PCI was preferred over CABG.(17) The 2011 guideline for PCI from the American College of Cardiology Foundation (ACCF), American Heart Association (AHA), and Society for Cardiovascular Angiography and Interventions also pointed out that for STEMI in which distal coronary flow was TIMI Grade < 3, PCI can be performed more rapidly and safely than CABG (class IIa recommendation).(18) Marso et al reported that the mortality during hospitalisation in the balloon angioplasty-only group (70%) and the stenting group (35%) differed significantly, proposing that stent implantation could improve the prognosis.(1) In a meta-analysis by Vis et al,(19) the 30-day mortality of patients receiving drug-eluting stent (DES) implantation was zero in the group without CS. In a study by Lee et al that studied 62 AMI patients with ULMCA lesions and DES implantation, DES was found to be safe and feasible.(20) In the present study, except for two patients who died immediately after angiography and two CABG patients, 51 patients received emergency PCI and 32 (71.1%) of the 45 stents were DES implantation. PCI with DES implantation should be the primary modality in these patients, with CABG used for ‘bail-out’ situations or when PCI is deemed not technically feasible.
In the present study, 94.5% of the patients received IABP implantation. The latest IABP in cardiogenic shock II study did not support the use of routine IABP implantation in CS patients receiving early revascularisation.(21) However, as recommended in the 2013 ACCF/AHA STEMI guideline, the use of IABP counterpulsation can be useful in patients with CS after STEMI who do not quickly stabilise with pharmacological therapy (Class IIa recommendation).(22) For emergency LMCA interventions, especially in patients with CS as a complication, IABP implantation may improve cardiac function and coronary perfusion. Parma et al also strongly recommended left ventricular assistance devices for these patients.(5) In addition, although there has been no supporting evidence, prophylactic implantation of IABP may be suitable for some patients who do not develop CS before revascularisation. It may help to prevent a sharp haemodynamic deterioration in a short period of time due to reperfusion injury after rapid restoration of blood flow, causing patients to lose the opportunity for further treatment.
The present study had its limitations. Firstly, due to the small sample size, it had a reduced power to identify predictors. Therefore, a multicentre registry study may be required in the future. Secondly, due to the low incidence of AMI due to ULMCA disease and the critical clinical conditions of these patients, it was difficult to perform a prospective, randomised study to explore the differences between various methods of revascularisation (e.g. CABG vs. PCI, bare-metal stents vs. DES) and between different haemodynamic support techniques (e.g. whether prophylactic IABP implantation or other cardiopulmonary assist devices are needed). Hence, we conducted a retrospective observational study. Thirdly, ten patients died before they could receive an echocardiography examination even though the LVEF of these patients might have been severely impaired, resulting in possible bias when the impact of LVEF on mortality was analysed.
In conclusion, the present study showed a high incidence of CS and mortality in AMI patients resulting from ULMCA lesions. CS, poor collateral blood flow and failure to restore final flow TIMI Grade 3 correlated with death during hospitalisation. CS was the only predictor of long-term overall mortality in these patients.


