Abstract
INTRODUCTION
Risk stratification in dilated cardiomyopathy (DCM) is imprecise, relying largely on echocardiographic left ventricular ejection fraction (LVEF) and severity of heart failure symptoms. Adverse cardiovascular events are increased by the presence of myocardial scarring. Late gadolinium enhancement (LGE) on cardiovascular magnetic resonance (CMR) imaging is the gold standard for identifying myocardial scars. We examined the association between LGE on CMR imaging and adverse clinical outcomes during long-term follow-up of Asian patients with DCM.
METHODS
Consecutive patients with DCM undergoing CMR imaging at a single Asian academic medical centre between 2005 and 2015 were recruited. Clinical outcomes were tracked using comprehensive electronic medical records and mortality was determined by cross-linkages with national registries. Presence and distribution of LGE on CMR imaging were determined by investigators blinded to patient outcomes. Primary endpoint was a composite of heart failure hospitalisations, appropriate implantable cardioverter-defibrillator shocks and cardiovascular mortality.
RESULTS
Of 86 patients, 64.0% had LGE (80.2% male; mean LVEF 30.1% ± 12.7%). Mid-wall fibrosis (71.7%) was the most common pattern of LGE distribution. Over a mean follow-up period of 4.9 ± 3.2 years, 19 (34.5%) patients with LGE reached the composite endpoint compared to 4 (12.9%) patients without LGE (p = 0.01). Presence of LGE, but not echocardiographic LVEF, independently predicted the primary endpoint (hazard ratio 4.15 [95% confidence interval 1.28–13.50]; p = 0.02).
CONCLUSION
LGE presence independently predicted adverse clinical events in Asian patients with DCM. Routine use of CMR imaging to characterise the myocardial substrate is recommended for enhanced risk stratification and should strongly influence clinical management.
INTRODUCTION
Risk stratification in non-ischaemic dilated cardiomyopathy (DCM) is imprecise, with current guidelines focusing on left ventricular ejection fraction (LVEF) and heart failure symptom status.(1-3) Markers for increased risk of sudden death, validated in ischaemic cardiomyopathy, perform less well for DCM.(4) Consequently, solely relying upon these parameters to risk stratify patients with DCM lowers the clinical efficacy and cost-effectiveness of primary prevention implantable cardioverter-defibrillator (ICD). On the other hand, at-risk patients with LVEF above the recommended cut-off may be excluded from ICD implantation.
A majority of sudden cardiac deaths are caused by ventricular arrhythmias, which in turn arise from re-entrant activity around heterogeneous myocardial scars.(5,6) Cardiovascular magnetic resonance (CMR) imaging is established as the gold standard for quantification and accurate identification of myocardial scarring by the detection of late gadolinium enhancement (LGE). Western cohorts of patients with DCM with LGE on CMR imaging have higher risks of adverse cardiac events, regardless of LVEF and heart failure symptom status.(7-10) In this study, we examined the characteristics of myocardial scarring in consecutive Asian patients with DCM and their association with long-term adverse clinical outcomes.
METHODS
The study population was extracted from a prospective registry of consecutive patients attending the National University Hospital, Singapore, for clinically indicated gadolinium-enhanced CMR imaging between 2005 and 2015. Patients were included if they had: (a) LVEF ≤ 50% on transthoracic echocardiography; (b) absence of significant obstructive stenosis on coronary angiography, normal stress echocardiography or myocardial perfusion scanning to exclude ischaemic heart disease; and (c) symptoms exceeding six weeks in duration before undergoing CMR imaging to avoid inclusion of patients with acute myocarditis.
Exclusion criteria were: (a) specific diagnosis of hypertrophic cardiomyopathy, sarcoidosis, arrhythmogenic right ventricular dysplasia and infiltrative cardiac disease, such as amyloidosis and cardiac lymphoma; and (b) patients who were not permanent residents or citizens of Singapore, as their mortality and cause of death could not be tracked by cross-linkage with national registries.
CMR imaging was performed using a 1.5T scanner (MAGNETOM Symphony or MAGNETOM Aera; Siemens Healthcare, Erlangen, Bavaria, Germany). Steady-state free precession breath-hold sequences (TE[echo time]/TR[repetition time] = 1.1/40 msec, 25 phases per cardiac cycle, matrix size 192 × 192) were used to acquire cine images in standard long-axis planes (slice thickness 8 mm) and in sequential 8-mm short-axis slices (with 2-mm gap) from the atrioventricular ring to the ventricular apex. LGE magnetic resonance imaging for the characterisation of myocardial scar was obtained as two-dimensional phase-sensitive inversion recovery sequences in identical planes to the cine images 10–15 minutes after the administration of intravenous gadolinium contrast (gadopentetate dimeglumine 0.2 mg/kg or gadobutrol 0.1 mL/kg), with each slice acquired per breath-hold. Inversion times were adjusted to null normal myocardium (typically 320–440 msec, matrix size 256 × 256). The LGE images were repeated in two separate phase-encoding directions to exclude artefacts. The scanning duration for each patient was around 45 minutes. Biventricular volumes and function were calculated using manual segmentation, using commercially available software (CMRtools; Cardiovascular Imaging Solutions, London, UK; or ADAS; Galgo Medical, Barcelona, Catalonia, Spain). Analyses of both the right and left ventricles were performed on a per slice basis by manual contouring of the endocardial and epicardial borders on the short-axis cine images, with contours manually drawn in end-diastole (start of R-wave) and in end-systole (smallest cavity area). Volumes were calculated based on the Simpson’s method. The ventricular trabeculations and papillary muscles were excluded from the blood pool during manual segmentation. CMR imaging analysis was performed by two of five investigators, who were blinded to patient clinical status and eventual outcomes. In the event of disagreement, a third observer was asked to adjudicate.
The primary endpoint was a composite endpoint of heart failure hospitalisations, appropriate ICD shocks and cardiovascular mortality. Clinical outcomes were tracked by study members who did not participate in CMR imaging analysis, using a comprehensive electronic medical records system (CPSS2, iHIS, Singapore). Cardiovascular mortality was determined on 26 April 2016 via linkages with datasets of the National Registry of Diseases Office (NRDO), Singapore. The NRDO was established by the Ministry of Health, Singapore, to collect data on selected major diseases and health conditions, such as cancer, acute myocardial infarction, stroke and kidney failure, and to track the mortality status of all Singapore citizens and permanent residents. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution’s human research committee (National Healthcare Group Domain Specific Review Board Approval number 2014/0424). Due to the use of a registered standing database and anonymised data, waiver of patient consent was granted by the ethics committee.
Continuous data and categorical variables were expressed as mean values ± standard deviation and percentages, respectively. Continuous and categorical variables were assessed using unpaired t-tests and Fisher’s exact tests, respectively. Pearson correlation was used to determine the relationship between two continuous variables. The log-rank test was used to compare Kaplan-Meier survival curves. To determine the association between clinical variables and the occurrence of primary endpoint, multivariate Cox proportional regression analyses were performed and results were expressed as hazard ratio (HR), with 95% confidence interval (CI). A p-value < 0.05 was considered to be statistically significant. All analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp, Armonk, NY, USA).
Mitral regurgitation was considered to be functional if anatomically normal mitral valve leaflets failed to coapt adequately due to global left ventricular remodelling secondary to DCM. The severity of functional mitral regurgitation was graded according to the American Society of Echocardiography guidelines.(11)
RESULTS
A total of 86 consecutive patients, who fulfilled the inclusion criteria, were identified. Patients were predominantly male (80.2%), with a mean echocardiographic LVEF of 30.1% ± 12.7%. LVEF, as determined by CMR imaging and echocardiography, was highly correlated (r = 0.78, p < 0.01). Baseline characteristics of all patients as well as the subgroups with and without LGE enhancement are listed in
Table I
Patient demographics.
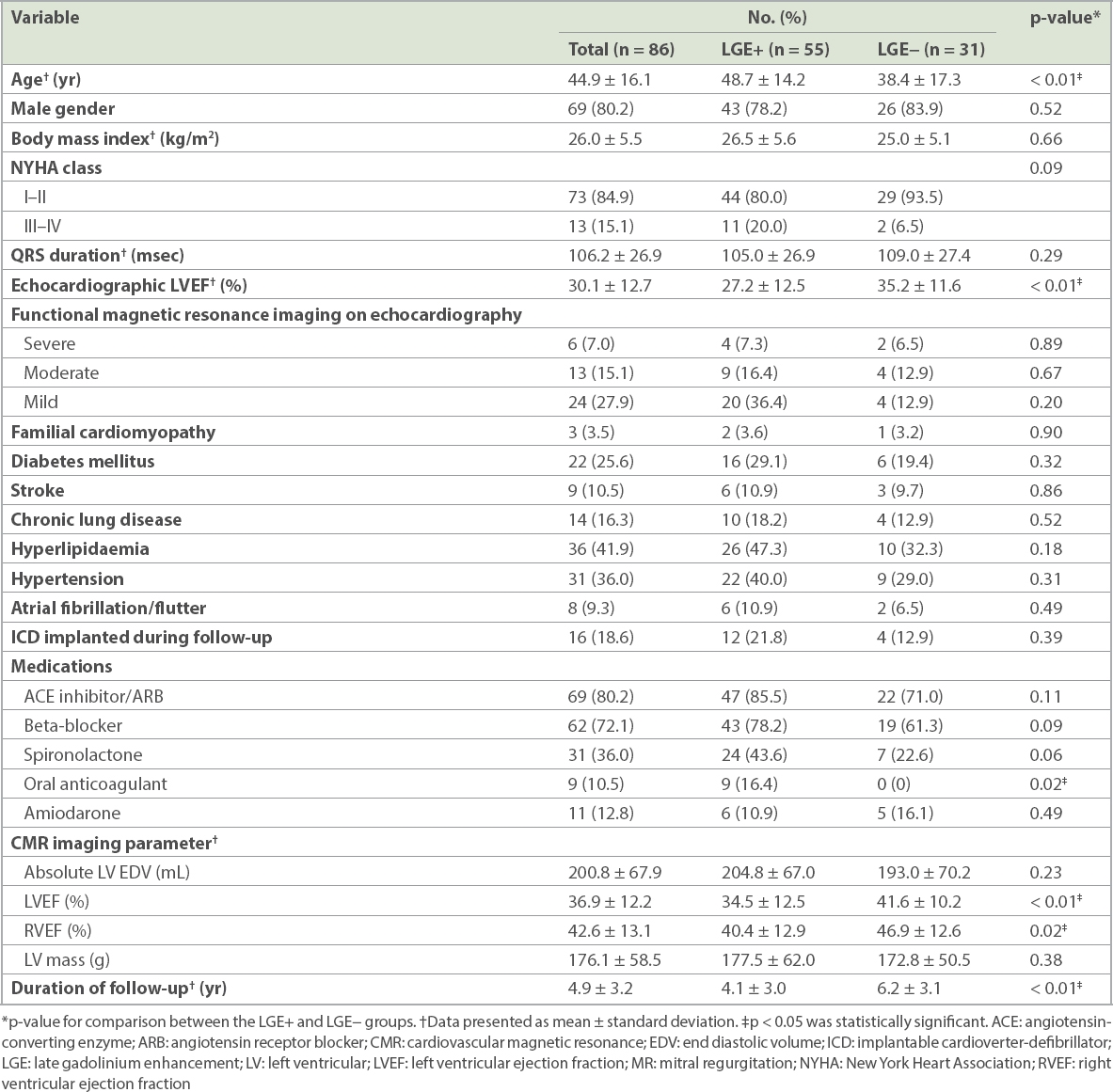
Fig. 1
Patterns of late gadolinium enhancement on cardiac magnetic resonance imaging of dilated cardiomyopathy show (a) epicardial scar, (b) mid-wall scar and (c) focal scarring.
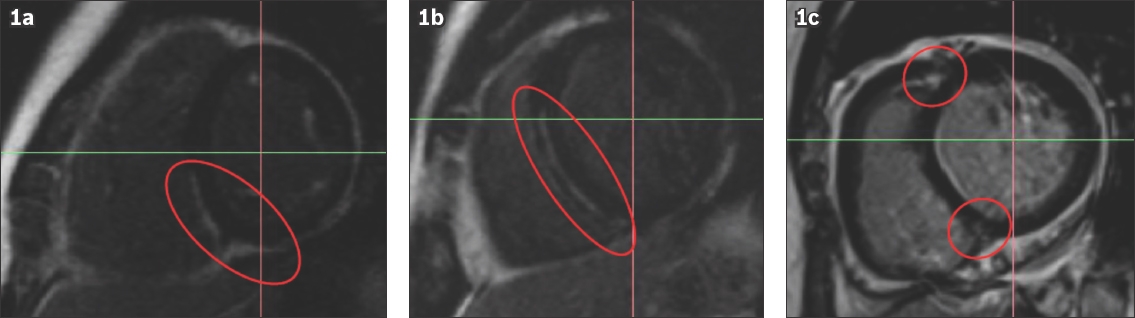
Fig. 2
Distribution of LGE based on AHA 17-segment model. AHA: American Heart Association; LGE: late gadolinium enhancement; LV: left ventricular; RV: right ventricular
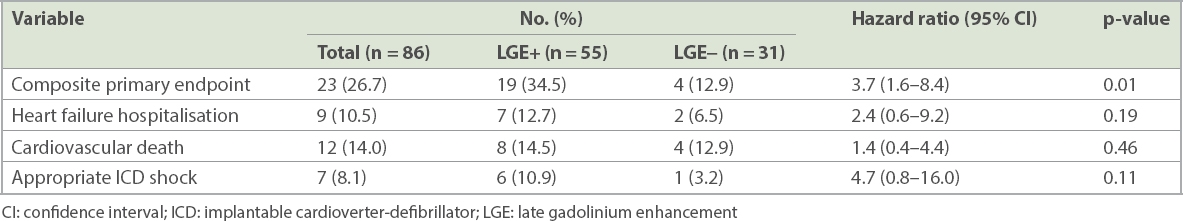
In total, 23 (26.7%) patients reached the composite endpoint, of which 19 (34.5%) patients were in the LGE+ group and 4 (12.9%) patients did not have LGE (LGE−; p = 0.01). Event-free survival at four years post CMR imaging was 96.8% in the LGE− group when compared to 59.7% in the LGE+ group (
Fig. 3
Kaplan-Meier survival curves for primary composite endpoint. CI: confidence interval; LGE: late gadolinium enhancement
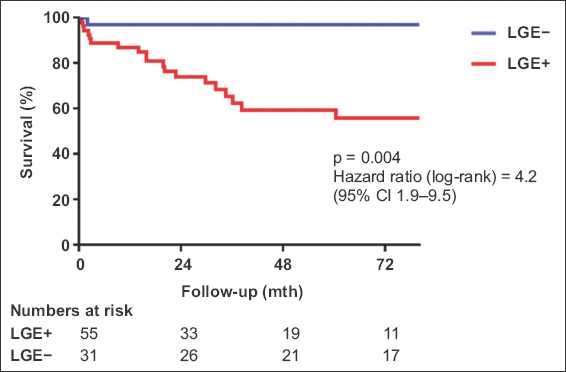
Table II
Clinical endpoints.
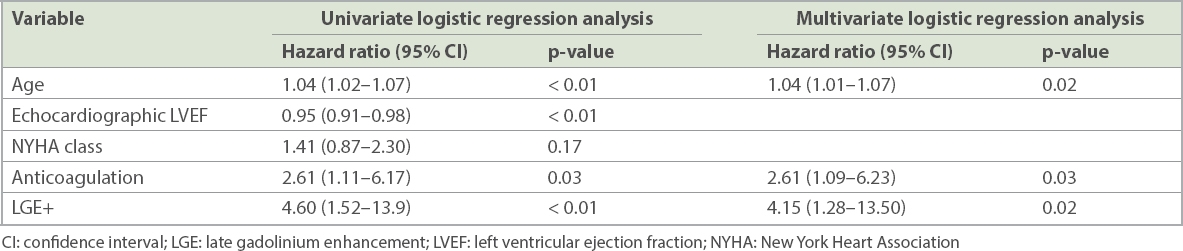
Increased age, higher New York Heart Association (NYHA) symptom status, anticoagulation therapy, lower echocardiographic LVEF and the presence of LGE were associated with increased likelihood of the occurrence of the primary endpoint (
Table III
Predictors of primary composite endpoint.
DISCUSSION
In this consecutive series of Asian patients with DCM undergoing CMR imaging, the presence of LGE independently and strongly predicted an increased risk of heart failure hospitalisation, appropriate ICD shocks and cardiovascular mortality.
While mid-wall fibrosis is the hallmark and most common pattern of LGE distribution in DCM, other distribution patterns include subendocardial scarring extending towards the epicardium and patchy foci, particularly around right ventricular and left ventricular septal insertion points.(12) All three patterns were seen in our cohort, with mid-wall fibrosis affecting 71.7% of patients. On postmortem analysis, these sites of LGE corresponded to macroscopic replacement fibrosis.(7) In a prospective longitudinal study of 472 patients with DCM undergoing CMR imaging, the presence and extent of mid-wall fibrosis was associated with a nearly threefold increased risk of all-cause mortality (annual mortality rates of 5.1% in patients with LGE when compared to 2.0% in patients without LGE) independent of LVEF and other conventional prognostic factors.(8) The predictive ability of LGE to predict adverse arrhythmic events was even stronger. In the same cohort, the presence of LGE was associated with a 5.2-fold increase in the likelihood of sudden death. These findings were further affirmed in the largest reported meta-analysis of 2,948 patients with DCM undergoing CMR imaging (44% of patients with LGE), whereby presence of LGE was associated with a relative 4.2-fold increase in composite adverse arrhythmic endpoints consisting of sudden cardiac death, aborted cardiac arrest, sustained ventricular arrhythmias and appropriate ICD shocks.(1) Interestingly, this association remained robust regardless of whether patients had severe or milder left ventricular dysfunction. In the subset of patients with primary prevention ICDs, the odds ratio of adverse arrhythmic events increased to 7.8 fold in patients with LGE compared to those without. The absence of LGE in patients with DCM was found to be associated with improved response to pharmacological and cardiac resynchronisation therapies.(13,14)
The demography of heart failure is significantly different between Caucasian and Asian cohorts, with the latter being younger but with higher rates of hypertension and diabetes mellitus.(15) While the prognostic value of detecting LGE on CMR imaging was established for Caucasian cohorts and meta-analyses performed, Asian case series from Japan and China, and now ours from Singapore, reaffirm the identification of LGE as a powerful tool for prognostication of Asian patients with DCM.(13,16,17) It is difficult to make further comparisons between Caucasian and Asian patient series, as there is significant heterogeneity in the profiles of patients reported in each series, inclusion criteria, choice of outcome measures and duration of follow-up.(1) Consequently, the proportion of patients with LGE on CMR imaging varies between 12% and 70%.
ICD implantation is indicated for patients with DCM who have LVEF ≤ 35%, and NYHA class II or III symptoms.(2,3) These recommendations were largely based on the positive results of the SCD-HeFT study, which demonstrated life-saving benefits of ICD therapy in a mixed patient population with ischaemic and non-ischaemic cardiomyopathy, with mild-to-moderate heart failure symptoms.(18) It has been well recognised from community cardiac arrest registries that 70% of patients with sudden death and premortem echocardiographic studies had LVEF that does not fulfil criteria for ICD.(19)
To date, to the best of our knowledge, no single randomised controlled trial of ICD therapy in patients with DCM has demonstrated statistically significant reduction in overall mortality, although significant reduction in arrhythmic mortality was seen in the DEFINITE and the recently completed DANISH studies.(20-22) However, in an updated meta-analysis of primary prevention studies that included DANISH, ICD implantation was still associated with a 23% reduction of all-cause mortality.(23) This was in contrast to the more convincing all-cause mortality reduction seen in primary prevention ICD trials that enrolled patients with ischaemic cardiomyopathy.(24) The reasons for this distinction include DCM having lower absolute mortality rates, better response to pharmacological and cardiac resynchronisation therapies, and, importantly, poor specificity of echocardiographic LVEF criteria to differentiate among patients with DCM who are most likely to benefit from ICD therapy. A prospective randomised study incorporating CMR imaging findings into the decision-making process for primary prevention ICD implantation in fully optimised patients with DCM is strongly warranted, with the aim to develop a more sensitive approach to selecting patients with DCM for ICD implantation. In the meantime, clinicians should be cognisant that the presence of LGE on CMR imaging, which is not incorporated into current guidelines, represents a significant risk marker for adverse clinical events, including arrhythmia and sudden death. An individualised risk-benefit assessment of ICD implantation should be considered for patients with DCM having LGE who do not fulfil current guideline criteria.
This study was not without limitations. The relatively small sample size limited the precision of point estimates of individual adverse clinical outcomes. The decision to refer patients with DCM for CMR imaging was left to physician discretion and our cohort was therefore subject to referral bias. There were no postmortem comparisons made to CMR imaging scans.
In conclusion, the presence of LGE on CMR imaging was an independent predictor of adverse clinical events for Singapore patients with DCM. Routine use of CMR imaging to characterise the myocardial substrate is recommended for enhanced risk stratification and may guide clinical management.
ACKNOWLEDGEMENTS
This study was supported by an unrestricted research grant provided by Boston Scientific and Galgo Medical to the Department of Cardiology, National University Heart Centre Singapore, Singapore.


