Abstract
INTRODUCTION
This study aimed to evaluate the proportion of young patients with type 1 diabetes mellitus (T1DM) who have myopia, as well as the risk factors associated with myopia in this group.
METHODS
In this cross-sectional study, patients aged < 21 years with T1DM for ≥ 1 year underwent a comprehensive eye examination. Presence of parental myopia, and average hours of near-work and outdoor activity were estimated using a questionnaire. Annualised glycosylated haemoglobin (HbA1c), defined as the mean of the last three HbA1c readings taken over the last year, was calculated. Multivariate analysis using genetic, environmental and diabetes-related factors was done to evaluate risk factors associated with myopia.
RESULTS
Of the 146 patients (mean age 12.5 ± 3.6 years) recruited, 66.4% were Chinese and 57.5% were female. Myopia (i.e. spherical equivalent [SE] of –0.50 D or worse) was present in 96 (65.8%) patients. The proportion of patients with myopia increased from 25.0% and 53.6% in those aged < 7.0 years and 7.0–9.9 years, respectively, to 59.2% and 78.4% in those aged 10.0–11.9 years and ≥ 12.0 years, respectively. Higher levels of SE were associated with lower parental myopia (p = 0.024) and higher annualised HbA1c (p = 0.011).
CONCLUSION
Compared to the background population, the proportion of myopia in young patients with T1DM was higher in those aged < 10 years but similar in the older age group. Myopia was associated with a history of parental myopia. Environmental risk factors and poor glycaemic control were not related to higher myopia risk.
INTRODUCTION
The aetiology of myopia is complex and multifactorial; each individual’s risk is determined by a complex interaction of genetic and environmental factors.(1) In several studies, myopia has been shown to be associated with higher socioeconomic status,(2) better educational levels,(3) several visually demanding occupations,(4,5) excessive near-work activity(6-9) and a lack of outdoor activity.(10-12) It is extremely common in Singapore, with recent population-based and cohort studies indicating that myopia (i.e. a spherical equivalent [SE] of –0.50 D or worse) is present in the following proportions in children and adolescents: 10.9%, 34.2%, 59.2% and 78.5% of those aged < 7.0, 7.0–9.9, 10.0–11.9 and ≥ 12.0 years, respectively.(11,13-22)
Over the last decade, it has been suggested that a carbohydrate-rich Western diet with a high glycaemic load may predispose one to juvenile-onset myopia.(23) It has also been hypothesised that chronic hyperglycaemia and hyperinsulinaemia may result in higher levels of free insulin-like growth factor (IGF)-1 and lower levels of IGF-binding protein 3, which in turn may result in unregulated scleral growth and myopia.(23) Thus, the question arises of whether young patients with poorly controlled diabetes mellitus (DM), which can result in hyperglycaemia, are more likely to have myopia. While there is some evidence that poor glycaemic control in young patients with DM is associated with myopia,(24) other studies suggest that there may be little or no association.(25,26) Therefore, the present study aimed to evaluate the proportion of young patients with type 1 DM (T1DM) who have myopia, as well as the risk factors associated with myopia in this group of young patients.
METHODS
This hospital-based, cross-sectional study was conducted in KK Women’s and Children’s Hospital (KKH), Singapore. All young patients with T1DM who were referred to the ophthalmology service in KKH for diabetic eye screening between June 2006 and January 2010 were included in this study. Patients who had T1DM for < 1 year or had any other ocular pathology (e.g. cataract or glaucoma) were excluded. The study was conducted in accordance with the tenets of The Declaration of Helsinki. The nature of the study was explained to the parents or guardians of the patients prior to the eye screening and informed written consent was obtained. The study was approved by the local ethics committee and funded by a KKH research fund (RAU/2006/107).
Information regarding the patients’ history of T1DM (i.e. age at diagnosis, duration of T1DM and level of annualised glycosylated haemoglobin [HbA1c] over the last one year) was obtained from the collaborating endocrinologists. Cycloplegic refraction was performed by trained optometrists 30 minutes after the instillation of three rounds of eye drops, at five-minute intervals. In the first round, one drop each of proparacaine 0.5%, tropicamide 0.5% and cyclopentolate 1% were instilled. In the second round, one drop each of phenylephrine 2.5% and cyclopentolate 1% were instilled; in the third round, one drop of cyclopentolate 1% was instilled. SE (sphere plus half cylinder) refraction was calculated. A slit-lamp examination was done to exclude the presence of cataracts and a fundus examination was performed to screen for diabetic retinopathy. The patients and their parents or guardians were also asked to complete a self-administered questionnaire detailing the presence of parental myopia and the amount of time the patient spent on near-work (i.e. reading, writing, using messaging on the phone or/and computer, playing mobile phone or handheld games, and watching television) and outdoor activity (i.e. outdoor physical education sessions in schools, sports and recreation activities). The amounts of time the patient spent in near-work and outdoor activities were estimated using this information.
Myopia was defined as an SE of –0.50 D or worse. Annualised HbA1c, defined as the mean of the last three HbA1c readings (which were spaced approximately four months apart), was calculated to assess the patients’ chronic glycaemic control over the last one year. Glycaemic control was categorised as (a) ideal, if HbA1c was < 6.0%; (b) optimal, if HbA1c was 6.0%–7.5%; (c) suboptimal, if HbA1c was 7.6%–9.0%; and (d) high-risk, if HbA1c was > 9.0%. The proportion of young patients with myopia among the T1DM patients was compared with the prevalence of myopia in the background Singapore population consisting of individuals from similar age groups. The prevalence rates were taken directly from cohort-, army- and population-based studies in Singapore published from 2001 onwards.(11,13-22) To calculate the average rate of prevalence, results were weighted according to the number of subjects participating in the study. The weighted average
where x is the percentage of myopic subjects in the study cohort of a particular study and w is the total number of subjects recruited in that study.(11,13-22) No attempt was made to adjust the results for important variables, such as gender or ethnicity, or to weigh the results according to differences in methods.
Statistical analysis was performed using the IBM SPSS Statistics version 19.0 (IBM Corp, Armonk, NY, USA). Demographic characteristics were presented as means and standard deviations for continuous variables, and percentages for categorical variables. As there was no difference in the mean SEs of the right and left eye (p = 0.923), only right-eye data was utilised for the univariate analysis. Categorical variables were analysed using the chi-square test and continuous variables were analysed using the independent sample t-test. A p-value < 0.05 indicated statistical significance. Multivariate analysis was performed using SE as a dependent variable and the following as independent variables: age, gender, ethnicity, age at diagnosis of T1DM, duration of T1DM, time spent on near-work and outdoor activities, history of parental myopia and annualised HbA1c. To enable data from both eyes to be used, the generalised estimating equation (GEE) method was used to account for correlation between both eyes. The model with the lowest quasi-likelihood under the independence model criterion is presented in the results.
RESULTS
A total of 146 patients with T1DM (age range 4.3–20.7 years) were included in the present study. Of these patients, 97 (66.4%) were Chinese, 22 (15.1%) were Malay, 18 (12.3%) were Indian and 9 (6.2%) were of other ethnicities; 84 (57.5%) were female (
Table I
Demographics of the young patients enrolled in the study (n = 146).
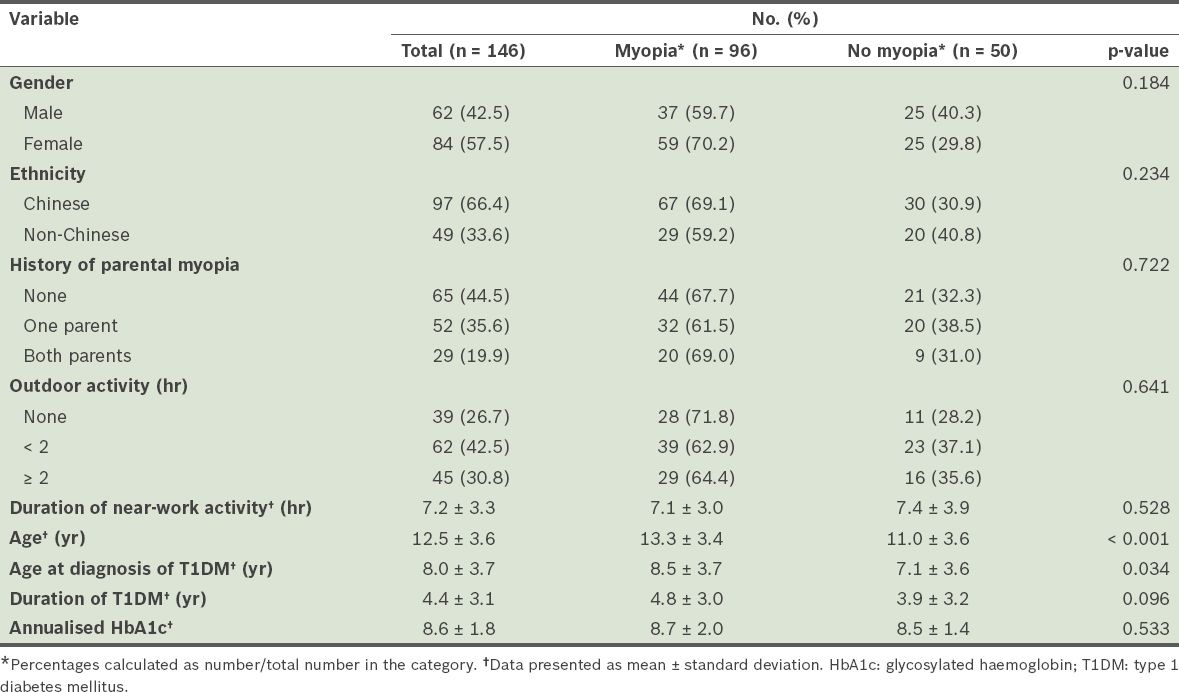
Fig. 1
Graph shows the age-specific proportions of myopia in patients with type 1 diabetes mellitus (T1DM) compared with the weighted average of myopia rates in the Singapore population. The numbers in parentheses show the number of patients with myopia in a specific age group within the study population.
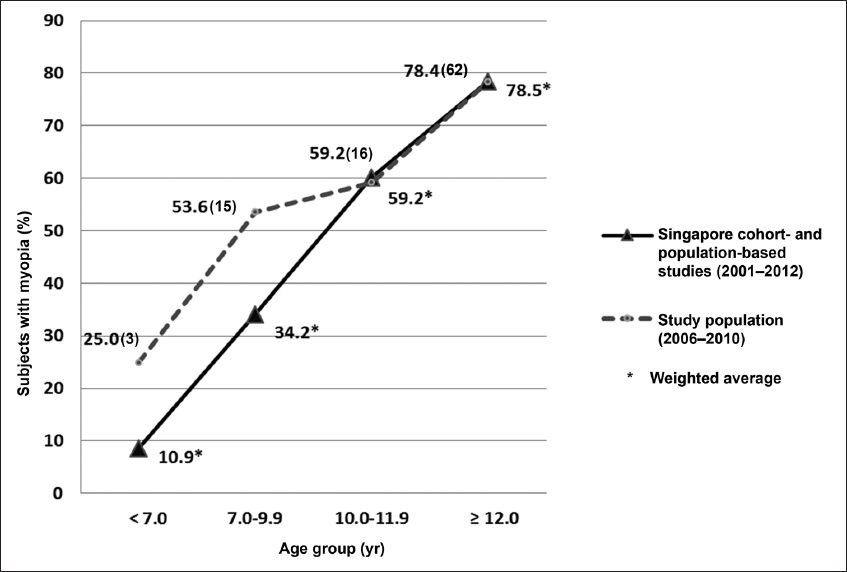
The results of the univariate analysis suggest that patients with myopia are more likely to be older (13.3 years vs. 11.0 years, p < 0.001) and diagnosed with T1DM later (8.5 years vs. 7.1 years, p = 0.034). The duration of T1DM tended to be longer in myopic patients (4.8 years vs. 3.9 years, p = 0.096), but the association was not statistically significant (
The results of the multivariable GEE analysis, which included the known risk factors of myopia (i.e. age, history of parental myopia, and number of hours spent on near-work and outdoor activities) and factors associated with T1DM (i.e. age at diagnosis of T1DM, duration of T1DM and annualised HbA1c), are shown in
Table II
Multivariate analysis of factors associated with spherical equivalent, using the generalised estimating equation model in patients with type 1 diabetes mellitus (T1DM) (n = 292).
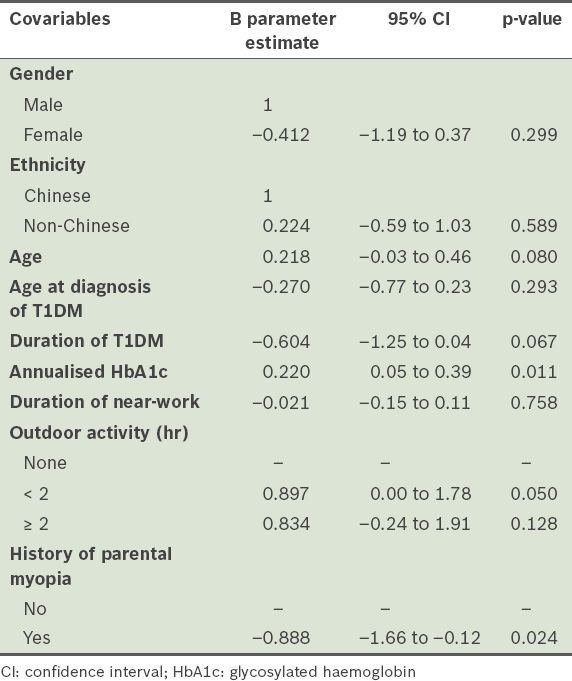
However, when the patients were categorised into the following groups: (a) no myopia (SE better than –0.50 D); (b) low myopia (–0.50 D to –2.50 D); and (c) high myopia (worse than –2.50 D), multiple logistic regression did not reveal a significant association between myopia and the level of annualised HbA1c (p = 0.136) after adjusting for age, history of parental myopia, duration of T1DM and environmental factors (e.g. hours spent in near-work and outdoor activities).
DISCUSSION
In the present study, the proportion of young patients with T1DM who had myopia was higher than the proportion in the background Singapore population of younger children (aged < 10 years). However, the proportion of patients with T1DM who had myopia in the older age group was similar to that in the background Singapore population of older children (≥ 10 years) (
Several studies have explored the relationship between refractive error and DM, with variable results. Mäntyjärvi et al, in a study involving children aged 9–16 years, found no difference in the prevalence of myopia among diabetic and non-diabetic individuals (36.1% vs. 29.3%, p > 0.30).(27) Johansen et al studied the relationship between SE and HbA1c at the time of examination, in young diabetic Dutch patients aged 7–15 years, and reported no association.(26) However, in a study conducted by Jacobsen et al, which involved older diabetic patients (aged 16–26 years), the authors found that there was a higher level of myopia in their study cohort as compared to that of the normal population (53.3% vs. 12.8%).(24) They also noted that poor glycaemic control for 2–3 months (HbA1c reading at baseline) was a potential risk factor for myopic shift; the relative risk of a myopic shift was 1.6 (95% confidence interval: 1.19–2.14) in those who had HbA1c levels above 8.8%.(24) In the present study, we noted an apparently conflicting finding: the proportion of myopia appeared to be higher in younger patients as compared to the background Singapore population, yet higher annualised HbA1c (i.e. long-term hyperglycaemia) was associated with a more positive SE rather than worsening myopia.
It has long been recognised that changes in the blood glucose level of patients with DM can produce changes in vision via altered refraction in the patients’ eyes. Both myopic(25,28-30) and hyperopic changes(31-34) have been observed in adolescents and adults with acute and chronic DM. Some of these changes may result from metabolic and structural changes within the lens.(30) However, Weimer et al suggested that an increase in lens thickness may also be offset by a concurrent decrease in equivalent refractive index and an increase in anterior lens curvature, resulting in little or no change in ocular refraction.(35) This could explain why no association was noted between SE and HbA1c in some of the studies.(26) However, the findings of the present study are supported by studies conducted on animal models, which showed that induction of DM and chronic hyperglycaemia in rabbits was associated with reduced axial growth and hyperopia.(36,37)
The underlying pathophysiology associated with T1DM is complex. In T1DM, hyperglycaemia is due to a lack of insulin production and is treated with subcutaneous insulin injections. HbA1c levels, which reflect the extent of glycaemic control, are higher in patients with poorer metabolic control. In children and adolescents with T1DM, HbA1c levels have been found to be inversely correlated to serum IGF-1 levels (r = –0.22, p < 0.005).(38,39) Poor metabolic control and a decrease in IGF-1 levels were also associated with a reduction in their growth and height.(40,41) This led us to speculate that poor glycaemic control may result in the retardation of the growth of the eyeball and more positive refraction due to lower IGF-1 levels.
The strengths of the present study include the use of cycloplegic refraction and annualised HbA1c values (rather than single HbA1c values). All patients aged < 21 years with T1DM who presented to the eye clinic between 2006 and 2010 were included in the present study. The study was not without limitations – it was cross-sectional in nature, the patients recruited were from a wide range of age groups and there were relatively fewer patients from the younger age groups. The age of onset and duration of T1DM among those recruited were also highly variable. Furthermore, the use of annualised HbA1c over the last one year may not fully reflect the patient’s variations in glycaemic control over time. Collection of biometric data (e.g. axial length, anterior chamber depth and lens thickness) and serum IGF-1 levels may have helped to provide insight into the mechanisms involved.
In summary, there may be an initial transient increase in the proportion of myopia among T1DM patients aged < 10 years. This may be due to the patients’ improved glycaemic control as endocrinologists attempt to manage the condition. No difference in refraction was noted in the older patients with T1DM, as compared to historical population norms, suggesting that T1DM may have minimal effect on refraction in the long term. We did not find any evidence to support the hypothesis that poor glycaemic control (i.e. hyperglycaemia) is associated with worsening myopia.


