Abstract
INTRODUCTION
Despite the widespread use of transient elastography for non-invasive assessment of liver fibrosis, the optimal cut-off liver stiffness measurement (LSM) values remain unclear. This study aimed to validate the optimal cut-off LSM values for significant fibrosis and cirrhosis in patients with chronic liver disease (CLD).
METHODS
Prospective multicentre data of CLD patients who underwent paired liver biopsy and LSM was analysed to determine the optimal cut-off LSM values for predicting significant fibrosis (METAVIR F ≥ 2) and cirrhosis (METAVIR F4). A high-quality cohort was selected by excluding those with failed LSM and invalid LSM readings.
RESULTS
Of the 481 patients recruited, 322 fulfilled the pre-defined quality criteria. CLD aetiology was chronic hepatitis B (CHB) in 49%, non-alcoholic steatohepatitis (NASH) in 16% and chronic hepatitis C (CHC) in 12%. Area under the receiver operating characteristic curve for LSM was 0.775 (95% confidence interval [CI] 0.724–0.826) for significant fibrosis and 0.810 (95% CI 0.738–0.882) for cirrhosis. Optimal cut-off LSM values were 9 kPa for significant fibrosis and 13 kPa for cirrhosis in the general cohort. Optimal cut-off LSM values were 9 kPa for significant fibrosis and 12 kPa for cirrhosis for both CHB and CHC, while the corresponding values for NASH were 11 kPa and 15 kPa.
CONCLUSION
Optimal cut-off LSM values should be selected based on disease aetiology. In Singapore, the optimal cut-off LSM values for CHB and CHC are 9 kPa for significant fibrosis and 12 kPa for cirrhosis. Optimal cut-off values for NASH require further validation.
INTRODUCTION
The assessment of liver fibrosis is integral to the management of chronic liver disease (CLD), as it provides prognostic information for therapeutic intervention to prevent the development of liver-related endpoints, such as cirrhosis, liver failure, liver cancer and death.(1) Although liver biopsy is regarded as the gold standard for fibrosis assessment, it is an invasive procedure that is associated with bleeding risk and is poorly accepted by patients. It is also limited by sampling error and inter-observer variability.(2,3) These factors have led to the development of non-invasive methods for assessment of liver fibrosis and cirrhosis. Of these, liver stiffness measurement (LSM) using transient elastography (TE) has been widely validated as a reliable predictor of fibrosis and cirrhosis in CLD.(4-14)
As a testament to the increasing acceptance of TE worldwide, clinical practice guidelines from various international liver associations have endorsed TE as an alternative to liver biopsy for liver fibrosis assessment to aid clinical decision-making in the management of chronic viral hepatitis.(15-17) However, the optimal cut-off LSM values reported in the literature are highly variable, ranging from 5.2 kPa to 9.7 kPa for significant fibrosis and 9.7 kPa to 22.7 kPa for cirrhosis.(17) This wide variability makes it difficult for the practising physician to interpret LSM results for clinical decision-making in the real-world setting. There are several reasons for the wide variability in cut-off LSM values reported in the literature. Firstly, most validation studies were performed in distinct homogeneous disease populations, resulting in different cut-off LSM values for different diseases. Meta-analysis of TE studies demonstrates that the accuracy of LSM is dependent on the prevalence of the condition in the specific population studied.(5) Secondly, many early studies of LSM were confounded by poor-quality TE readings, inadequate operator experience, obesity, elevated transaminases and suboptimal liver biopsy samples,(18-23) including the first validation study of TE in Singapore.(12) Recent clinical practice guidelines have emphasised that the correct interpretation of LSM in clinical practice must consider the quality of the LSM.(15-17) Attention must also be given to the following: interquartile range/median value (IQR/M); serum transaminase levels; use of the appropriate probe (extra-large [XL] probe if skin-to-capsule distance > 25 mm); and absence of clinical conditions known to affect liver stiffness when interpreting LSM.(17)
In most hospitals in Singapore, TE is now routinely used to aid physicians in the management of CLD. There is, therefore, an important need to define the appropriate cut-off LSM values that are validated for our local population, based on the prevalence and spectrum of liver diseases that are unique to the Singapore population. Furthermore, these cut-off values must be validated using reliable, high-quality LSM criteria. To this end, we designed a prospective, multicentre study of high-quality, biopsy-paired TE measurements with the primary aim of defining the optimal cut-off LSM values that are validated in our specific patient population in Singapore for the diagnosis of liver fibrosis and cirrhosis. The secondary aim was to define the optimal cut-off LSM values for specific aetiologies of liver disease.
METHODS
We reviewed prospectively collected data of biopsy-paired TE from two tertiary hepatology centres in Singapore. This data was collected from the time TE was available in the individual centres. Consecutive patients who underwent liver biopsy and LSM were recruited for the study. Liver biopsies were performed for clinical indications, including staging of fibrosis for therapeutic decisions in patients with chronic viral hepatitis, prognostication in patients with non-alcoholic steatohepatitis (NASH), and diagnostic confirmation of patients with suspected autoimmune hepatitis, primary biliary cirrhosis or unexplained sustained abnormalities in liver function. Patients with decompensated liver cirrhosis and those with hepatocellular carcinoma were excluded from the study. The institutional review boards of the respective institutions approved the study.
Demographic, biochemical, anthropometric and clinical data of each patient was prospectively collected prior to the liver biopsy. Variables recorded included age, gender, race, aetiology of liver disease, body mass index (BMI), baseline liver function tests, serum creatinine and haematological indices. Liver biopsies were performed via the percutaneous, transjugular or surgical routes based on clinical indication and standard clinical practice. Liver specimens were stained according to standard histopathology practice for the assessment of liver fibrosis. Severity of fibrosis was staged by an experienced histopathologist from each centre and standardised across the two centres using the METAVIR scoring system.(24)
LSM was performed by experienced operators who had each completed more than 100 examinations. Each measurement was performed after a three-hour fast over the right lobe of the liver, with the patient lying in the supine position with the right arm abducted. At least ten valid LSM readings were obtained and the median LSM reading was recorded. Inability to obtain any valid readings was considered an LSM failure. Measurements with < 10 readings, < 60% success rate and IQR > 30% of the median LSM (i.e. IQR/M > 0.3) were deemed to be invalid. Where the XL probe was available, it was used for LSM in patients with skin-to-liver capsule distance ≥ 25 mm or BMI > 28 kg/m2.
We adhered to the recommendations of recently published clinical guidelines,(17) restricting the study population to a high-quality cohort by excluding known confounders. All LSM failures, invalid LSM readings, patients with BMI > 30 kg/m2, liver biopsy specimens < 15 mm in length and patients with significant transaminitis (alanine aminotransferase [ALT] > 5 × the upper limit of normal [ULN]) were excluded. This limited the number of falsely elevated LSM values and provided the most reliable cut-off LSM values for clinical use.
The primary outcome of the study was to define the optimal LSM values for the diagnosis of significant liver fibrosis (METAVIR ≥ F2) and cirrhosis (METAVIR F4) in a selected high-quality cohort. The diagnostic performance of TE was assessed by the C-statistic (with 95% confidence interval [CI]), which was estimated from the area under the receiver operating characteristic curve (AUROC), and the result was compared against the aspartate aminotransferase-to-platelet ratio index (APRI), a well-validated serum marker of fibrosis.(25) We reported optimal LSM values for the diagnosis of significant fibrosis and cirrhosis based on maximal diagnostic accuracy (maximum sum of sensitivity and specificity), maximal sensitivity (sensitivity > 90%) and maximal specificity (specificity > 90%). We then examined the optimal cut-off LSM values for individual aetiologies of liver disease based on a similar methodology. Qualitative and quantitative differences among groups were compared using chi-square test (or Fisher’s exact test where applicable) and Student’s t-test (including one-way analysis of variance where applicable), respectively. A two-sided p-value < 0.05 was deemed to be statistically significant. Data in the text and tables was represented as mean ± standard deviation or number (percentage), unless otherwise specified.
RESULTS
A total of 481 patients were recruited from two tertiary hospitals in Singapore – National University Hospital and Singapore General Hospital. All subjects underwent liver biopsy, which was paired with TE performed within three months of the biopsy. From the overall cohort, we excluded those with LSM failures (n = 24), invalid LSM readings (n = 22), invalid biopsy specimens (n = 75), BMI > 30 kg/m2 (n = 22) and ALT > 5 × ULN (n = 16), resulting in a final cohort of 322 patients who fulfilled the criteria for high-quality TE measurements. The baseline clinical characteristics of the study cohort are summarised in
Table I
Baseline characteristics of the study cohort (n = 322).
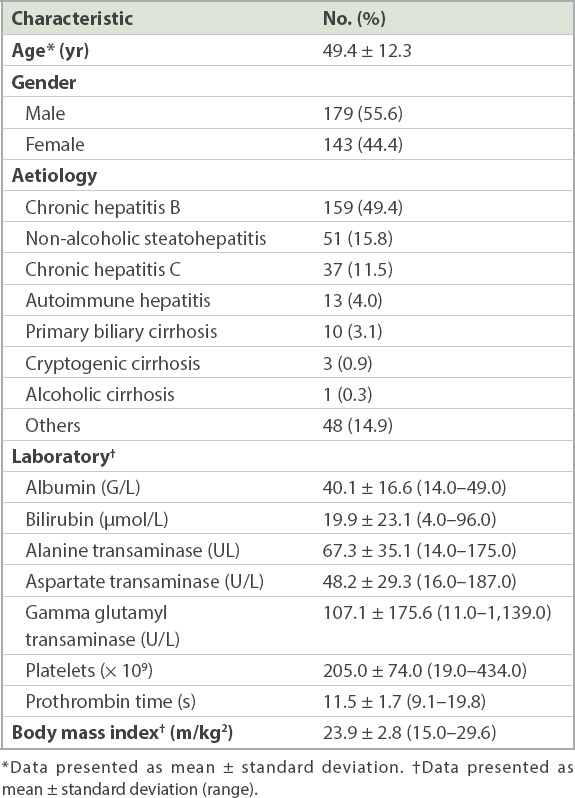
Liver biopsy was performed via the percutaneous route in 88.8% of patients, transjugular route in 5.2% and surgical route in 5.8%. Mean specimen length was 24.1 ± 6.9 mm. METAVIR data was not reported in ten patients, and was thus only available in 312 patients. METAVIR fibrosis grades were evenly distributed among the various stages (
Table II
LSM values according to METAVIR fibrosis groups (n = 312).
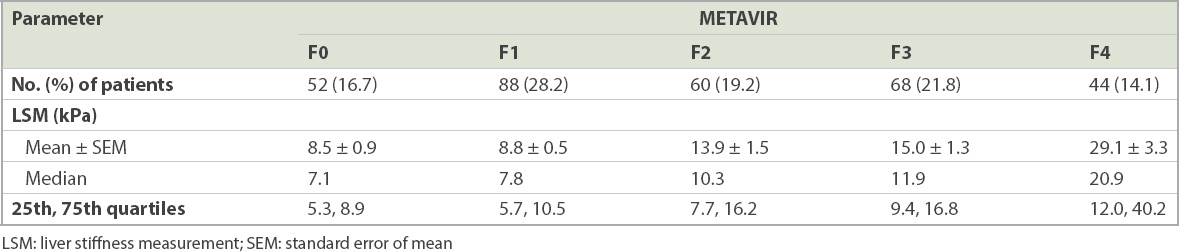
Results of TE were available for all 322 patients. Median LSM value was 9.7 kPa (range 3.4–75.0 kPa), while mean IQR was 2.1 ± 2.3 kPa and median IQR/M was 0.15 ± 0.07 kPa. The distribution of LSM according to METAVIR fibrosis groups is shown in
Fig. 1
Graph shows the range of liver stiffness measurement (LSM) values according to the METAVIR fibrosis groups. SE: standard error
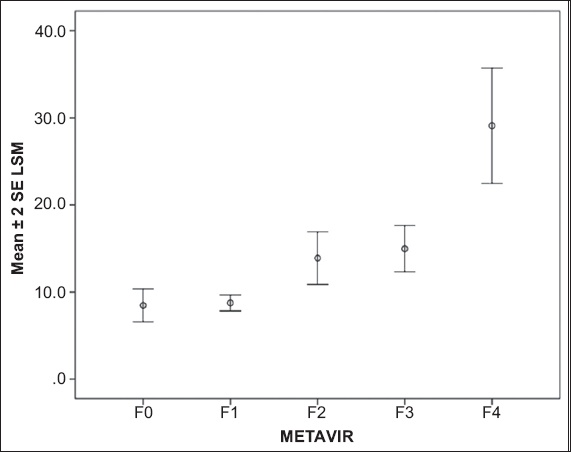
Fig. 2
Graphs show the area under the receiver operating characteristic curve (AUROC) of liver stiffness measurement for the prediction of (a) significant fibrosis (METAVIR ≥ F2) and (b) cirrhosis (METAVIR F4). CI: confidence interval
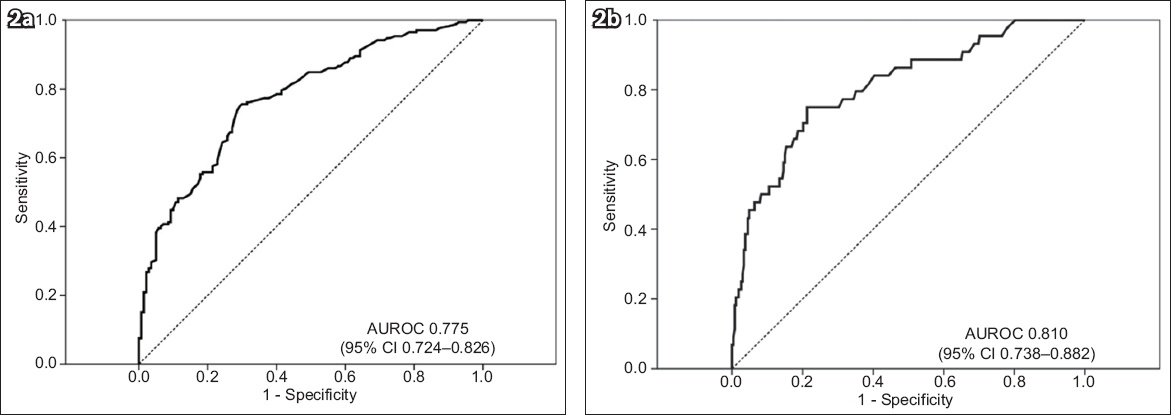
Table III
Optimal liver stiffness measurement values for the prediction of significant fibrosis and cirrhosis.
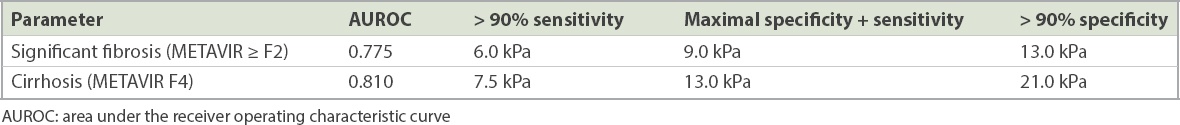
AUROC for TE diagnosis of cirrhosis (METAVIR F4) was 0.810 (95% CI 0.738–0.882, p < 0.001;
For the prediction of significant fibrosis and cirrhosis, TE was found to perform better than APRI. For the prediction of significant fibrosis, AUROC of TE was 0.775 (95% CI 0.724–0.826) vs. 0.584 (95% CI 0.521–0.648) for APRI. For the prediction of cirrhosis, AUROC of TE was 0.810 (95% CI 0.738–0.882) compared to 0.662 (0.578–0.746) for APRI.
Next, we evaluated the AUROC and optimal LSM values for assessment of significant fibrosis and cirrhosis in individual aetiologies of liver disease (
Patients with chronic hepatitis B (CHB) made up a majority (n = 159) of the cohort. Among CHB patients, 59.7% had significant fibrosis and 12.6% had cirrhosis. Optimal cut-off LSM values of 9 kPa for significant fibrosis and 12 kPa for cirrhosis allowed for correct classification in 70.5% and 77.6% of CHB patients, respectively. The optimal cut-off LSM values for chronic hepatitis C (CHC) were similar at 9 kPa for significant fibrosis and 12 kPa for cirrhosis, which correctly classified 81.1% and 70.3% of patients, respectively.
Table IV
Optimal cut-off LSM values for the diagnosis of significant fibrosis (METAVIR ≥ F2) and cirrhosis (METAVIR F4) in individual aetiologies of chronic liver disease.
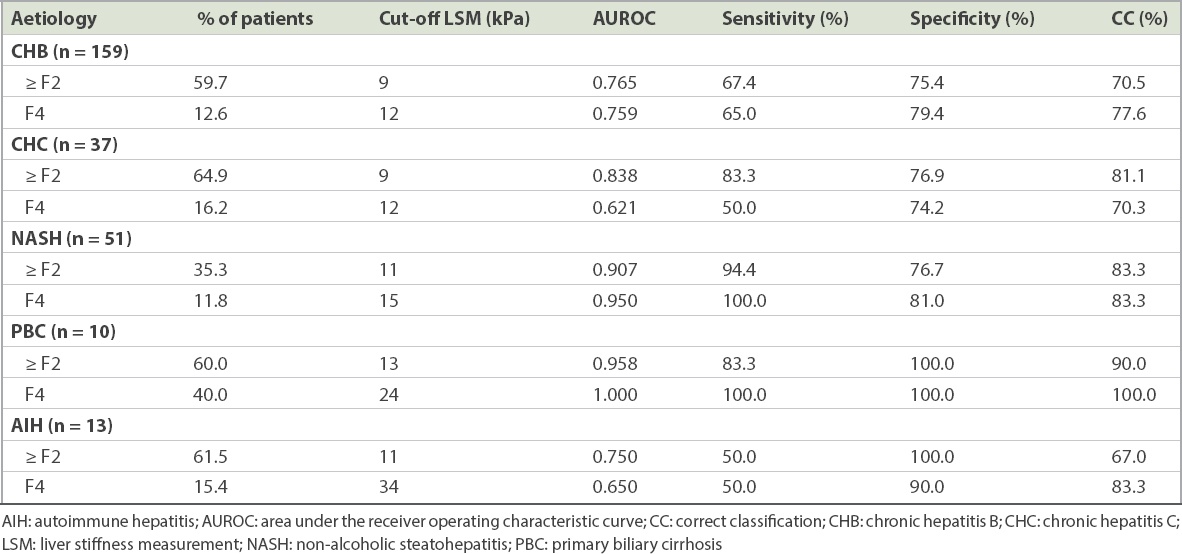
In the NASH cohort, the performance of TE for the prediction of significant fibrosis and cirrhosis fared better than that for the viral hepatitis cohort; the optimal cut-off LSM values were higher. An LSM value of 11 kPa was the optimal cut-off for the prediction of significant fibrosis with a high AUROC of 0.907, sensitivity of 94.4%, specificity of 76.9% and a correct classification rate of 83.3%. The optimal cut-off LSM value for the prediction of cirrhosis was 15 kPa with an AUROC of 0.950, sensitivity of 100.0%, specificity of 81.0%, and a correct classification rate of 83.3%.
Assessments of the performance of TE in autoimmune hepatitis (n = 13) and primary biliary cirrhosis (n = 10) were limited by the small numbers. For both of these aetiologies, although the optimal cut-off LSM values for prediction of significant fibrosis and cirrhosis were notably higher compared to that for viral hepatitis, the small sample size precluded any meaningful analysis.
DISCUSSION
This is the first multicentre, quality-controlled, biopsy-paired study of TE performed in Singapore. Our study demonstrated an optimal LSM of 13 kPa for the diagnosis of liver cirrhosis in the overall study cohort with mixed aetiologies. This locally validated cut-off level for non-invasive diagnosis of liver cirrhosis is consistent with those reported in previous publications, which ranged from 10.0 kPa to 14.8 kPa.(17) A large meta-analysis assessing the performance of TE reported an optimal cut-off LSM of 13 kPa for the diagnosis of cirrhosis, independent of aetiology of liver disease;(5) this result is congruent with the optimal cut-off LSM in our study, and thus validates the excellent performance of TE for non-invasive diagnosis of cirrhosis.
The clinical importance of non-invasive diagnosis of cirrhosis is that it enables reliable detection of early liver cirrhosis in patients with chronic liver disease. Early diagnosis of cirrhosis heralds the need for gastroscopy for varices screening, regular surveillance for hepatocellular carcinoma (HCC), and active treatment of the underlying liver disease to prevent progression to decompensation.(26) Conversely, reliable exclusion of cirrhosis reduces unnecessary invasive endoscopy, cuts down the frequency of HCC surveillance, and may even delay the need for expensive long-term antiviral treatment for CHB. These factors impact patient care by lowering healthcare costs and the utilisation of healthcare resources. Therefore, TE plays a useful clinical role for reliable exclusion of liver cirrhosis, as it performs particularly well to rule out cirrhosis with its high NPV of 94% irrespective of disease aetiology. TE is now accepted as the most accurate non-invasive method for diagnosing liver cirrhosis.(27) Although ultrasonography (US) is widely used to diagnose liver cirrhosis, there are concerns regarding the overestimation of cirrhosis based on the US finding of coarse echogenicity alone, leading to a false positive diagnosis of liver cirrhosis.(28) In patients with a low pre-test probability of cirrhosis (normal liver function, platelet counts and spleen size) who are diagnosed with early cirrhosis on US, we recommend seeking confirmation with TE. An LSM value < 13 kPa should caution the physician against labelling such patients as cirrhotic. These patients may benefit from liver biopsy to confirm the diagnosis of cirrhosis.
In contrast to cirrhosis, the cut-off LSM value for significant fibrosis varies depending on the aetiology of the underlying liver disease.(5) In the Singapore population, where CHB is the predominant aetiology, a cut-off LSM value of 9 kPa provides the highest accuracy for the diagnosis of significant fibrosis, allowing correct classification in 73% of the study cohort. Disease-specific cut-off LSM values can thus be applied in clinical practice to help physicians make management decisions for patients with CHB. In CHB, detection of significant fibrosis guides the decision to commence antiviral treatment to prevent progression to cirrhosis.(15,26) This decision has important economic implications since many patients, especially those with hepatitis B ‘e’ antigen (HBeAg)-negative disease, will require long-term continuous therapy with nucleoside analogues. Conversely, reliable exclusion of significant fibrosis provides the physician with the confidence to delay the initiation of unnecessary and expensive treatment for selected patients. For example, if a 40-year-old Chinese man with HBeAg-negative CHB, ALT 1–2 × ULN and a low-to-moderate hepatitis B virus DNA level (2,000–20,000 IU/mL) has an LSM value < 6 kPa, he is unlikely to have significant fibrosis, and can thus be monitored. However, if he has an LSM value > 9 kPa, he is likely to have significant fibrosis, which requires treatment to be commenced. Liver biopsy can be avoided in such patients, unless there is a discrepancy between the LSM value and clinical impression. Based on the data from our study, liver biopsy can be avoided in more than 70% of CHB patients.
In CHB, fluctuating ALT levels must be taken into account when interpreting LSM values.(29) In our study, we specifically excluded patients with ALT > 5 × ULN. Hence, the recommended cut-off LSM value of 9 kPa should only be applied to CHB patients with ALT within this range. CHB patients with ALT > 5 × ULN should undergo either repeat TE when the ALT falls below this threshold or liver biopsy for accurate staging of fibrosis. TE algorithms incorporating elevated ALT have been developed, but they have not been widely validated clinically.(30,31)
The relatively small number of patients with CHC and NASH in our study limits the ability to make definitive conclusions regarding the optimal cut-off LSM values for diagnosis of significant fibrosis in these conditions. Nonetheless, the results from our study are consistent with those of the published literature, demonstrating that the optimal cut-off LSM values for CHC are similar to those for CHB (i.e. 9 kPa for significant fibrosis and 12 kPa for cirrhosis).(17) Although the current availability of highly effective, direct-acting antiviral agents has made the diagnosis of significant fibrosis less essential in the management of CHC, TE still has a relevant role to play in patient selection for prioritisation of treatment. CHC treatment should be prioritised for patients with advanced fibrosis or cirrhosis, justified for those with moderate fibrosis, and individualised for those with no or mild fibrosis.(32) In addition, TE has a role in the non-invasive diagnosis of liver cirrhosis in CHC patients, and this will have an impact on the choice and duration of the antiviral treatment.
There is currently no pre-existing local data on the reliability of TE in NASH. From our cohort of 51 biopsy-proven NASH patients, we observed that TE was highly reliable for the prediction of significant fibrosis and cirrhosis in NASH patients with AUROCs of 0.907 and 0.950, respectively. Interestingly, the optimal cut-off LSM values of 11 kPa for significant fibrosis and 15 kPa for cirrhosis are higher in NASH as compared to CHB and CHC, and they are also higher compared to other studies conducted on TE in NASH.(14,33) Our results may, however, be confounded by the small sample size, lower prevalence of significant fibrosis in the NASH cohort, and the use of the METAVIR scoring system for grading fibrosis. The METAVIR scoring system was developed and validated for portal-based fibrosis such as viral and alcoholic liver disease, whereas NASH fibrosis should be graded using the NASH-Clinical Research Network grading system. Nonetheless, these interesting results raise the need for larger studies that focus on validating the optimal cut-off LSM values for NASH in Singaporean patients. This is clinically important because the local incidence of NASH is on the rise, which underscores the necessity of a reliable non-invasive method of assessing fibrosis in NASH.
The strength of the present study lies in the selection of a cohort that meets the recommended quality criteria for TE assessment.(17) Firstly, we selected patients with high-quality TE measurements, with TE performed by experienced operators using standardised methods that strictly adhered to the manufacturer’s recommendations. Secondly, we controlled for potential confounders, including elevated BMI, ALT > 5 × ULN and inadequate biopsy samples, which are all known to reduce the reliability of LSM. Thirdly, the inclusion of patients from more than one institution reduces the limitations of a single-site study and improves the reliability of our findings. In a sub-analysis comparing the results of the two separate sites, the AUROCs and optimal LSM values for significant fibrosis and cirrhosis were found to be similar.
The main limitation of our study is that it was performed in tertiary centres, which introduces a spectrum bias where study subjects are more likely to have more severe fibrosis. This raises the question of whether the cut-off LSM values in our study can be applied to the use of TE in primary care settings. To answer this question, we have presented a range of cut-off LSM values based on maximal sensitivity, maximal diagnostic accuracy and maximal specificity to aid the clinician in selecting the appropriate cut-off based on the clinical indication for TE (
In conclusion, the overall cut-off LSM values of 9 kPa and 13 kPa provide reasonable accuracy for the prediction of significant fibrosis and cirrhosis, respectively, in the Singapore population. However, the selection of an optimal cut-off LSM value for clinical use should be based on disease-specific cut-off values that vary among different aetiologies of CLD. In chronic viral hepatitis, the optimal cut-off LSM values of 9 kPa (range 6–12 kPa) and 12 kPa (range 6.5–16 kPa) are identified for the prediction of significant fibrosis and cirrhosis, respectively. The optimal cut-off LSM values for NASH still require further validation. We hope that the results of this prospective, multicentre study in a high-quality cohort will help to standardise the interpretation of TE in Singapore.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the contributions of all the physicians in the Department of Gastroenterology and Hepatology, Singapore General Hospital and the Division of Gastroenterology and Hepatology, National University Hospital. We also acknowledge the contributions of the technicians who performed the LSM. We thank Dr Jeffrey Ngu Jing Hieng and Dr Roy Soetikno from the Department of Gastroenterology and Hepatology, Singapore General Hospital, for reviewing the manuscript prior to submission. This study was funded by a grant from the Ministry of Health, Singapore (MOH RF Grant RF/HSDP10CS01S).


