Abstract
INTRODUCTION
We aimed to determine the optimal placement of electrodes for neuromuscular electrical stimulation (NMES) for post-stroke dysphagia therapy.
METHODS
31 patients with post-stroke dysphagia were randomised to three groups according to NMES electrode placement. In Group A (n = 10), two pairs of electrodes were attached horizontally on the suprahyoid and infrahyoid muscles. In Group B (n = 11), one pair of electrodes was attached horizontally on the suprahyoid muscles while the other was attached vertically on the infrahyoid muscles. In Group C (n = 10), the electrodes were attached vertically, with one pair above the hyoid bone and the other above the cricoid cartilage. All patients received rehabilitation treatment via NMES combined with effortful swallowing training five times weekly for four weeks. The effect of NMES electrode placement was assessed in terms of the Functional Dysphagia Scale (FDS) and Dysphagia Outcome and Severity Scale (DOSS) scores.
RESULTS
Group A showed significantly greater improvement than Group B in overall FDS (p = 0.009) and pharyngeal-phase FDS (FDS-P; p = 0.005) scores. Group A also showed significant improvement when compared with Group C in overall FDS (p = 0.001) and FDS-P (p = 0.001) scores.
CONCLUSION
Horizontal placement of the NMES electrodes on the suprahyoid and infrahyoid muscles for the treatment of post-stroke dysphagia by NMES combined with effortful swallowing was more effective than the horizontal and vertical placement of electrodes on the suprahyoid and infrahyoid muscles, respectively, and their vertical placement above the hyoid bone and cricoid cartilage.
INTRODUCTION
Swallowing dysfunction, or dysphagia, is known to occur in up to 76% of stroke patients. Patients with post-stroke dysphagia develop complications, such as malnutrition, dehydration and pneumonia, which can delay functional recovery. Pneumonia accounts for at least 10% of post-stroke deaths within 30 days of hospitalisation for stroke.(1-4) Therefore, early detection and treatment of dysphagia is crucial for recovery.
Conventional dysphagia treatment consists of oropharyngeal exercises, compensation manoeuvres and oropharyngeal stimulation.(5) Singh and Hamdy recommended that standard treatment for post-stroke dysphagia includes compensatory manoeuvres, such as postural adjustment, Mendelsohn manoeuvre, supraglottic swallowing and effortful swallowing.(6) Among these, effortful swallowing was shown to significantly decrease the residue in the oral cavity, increase oral pressure and improve hyoid bone elevation during swallowing in healthy adults.(7)
Recent studies have reported that stimulating the pharyngeal muscles using neuromuscular electrical stimulation (NMES) was effective for improving swallowing function.(8-10) By stimulating the mylohyoid, geniohyoid and thyrohyoid muscles, Burnett et al achieved laryngeal elevation in healthy volunteers.(8) Leelamanit et al reported that synchronised electrical stimulation of the thyrohyoid muscle during swallowing has a therapeutic effect in patients with dysphagia.(9) A retrospective study of NMES reported that NMES therapy appeared to be beneficial for patients with mild-to-moderate dysphagia.(10)
In previous studies on the effect of NMES therapy for post-stroke dysphagia, the following three therapeutic methods, according to NMES placement, were most commonly used.(10-16) Firstly, two pairs of electrodes were attached horizontally on the suprahyoid and infrahyoid muscles.(10-12) Secondly, one pair of electrodes was attached horizontally on the suprahyoid muscles while the other was attached vertically on the infrahyoid muscles.(10,13,14) Lastly, two pairs of electrodes were attached vertically above the hyoid bone and cricoid cartilage.(10,15,16) However, there is no clinical consensus on which NMES electrode placement is most effective for dysphagia therapy.
Therapeutic methods that combine NMES with effortful swallowing training have been proven to be beneficial for swallowing function improvement.(17) In the present study, we hypothesised that electrode placement may influence the therapeutic effect of NMES combined with effortful swallowing in patients with post-stroke dysphagia. The study was designed to apply the therapeutic methods that combined NMES with effortful swallowing training with the three most commonly used placements of electrodes. The study objective was to determine the most effective NMES electrode placement for the treatment of post-stroke dysphagia by NMES combined with effortful swallowing training.
METHODS
31 patients with post-stroke dysphagia were enrolled for this study, which was conducted at the Department of Rehabilitation Medicine, Kyungpook National University Hospital, Daegu, South Korea, between 1 January 2016 and 30 September 2017. The inclusion criteria were: stroke confirmed on computed tomography or magnetic resonance imaging; post-stroke dysphagia confirmed on videofluoroscopic swallowing study (VFSS); and sufficient language and cognitive function to perform effortful swallowing training. The exclusion criteria were: other neurologic diseases and medical condition(s) that represented a contraindication to NMES, such as cardiac pacemaker and epilepsy. This research was approved by the institutional review board of Kyungpook National University Hospital (approval no. KNUMC_15-1021). All patients included in the study provided informed consent prior to participation.
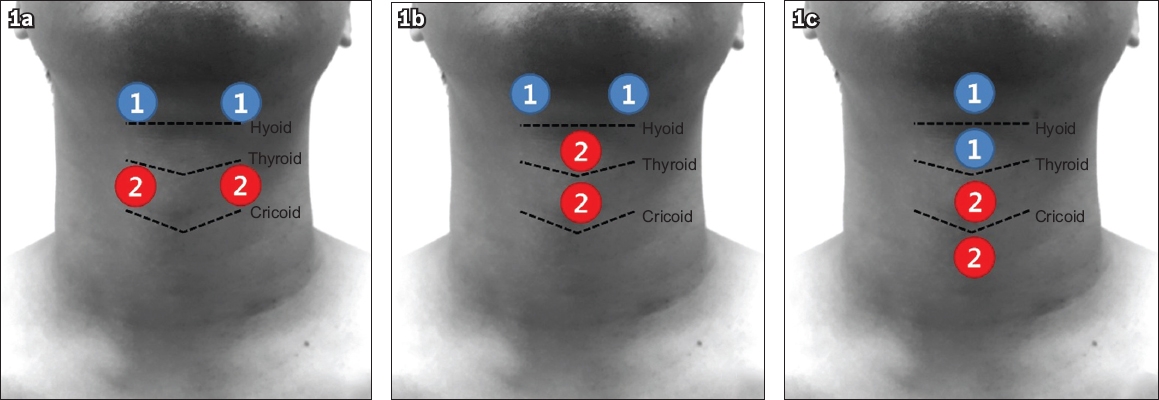
This study was designed as a single-blind, randomised controlled trial with a blinded observer. A computer-generated randomisation sequence and an automated assignment system were used for allocation. 31 patients with post-stroke dysphagia were randomly allocated into the three groups according to NMES electrode placements. In Group A, one pair of electrodes was attached on the suprahyoid muscles horizontally just above both ends of the hyoid bone based on palpation of the hyoid bone. A second pair of electrodes was attached horizontally on the infrahyoid muscles inferior to the hyoid bone for these patients. In Group B, the edge of the hyoid bone was first detected by palpation and one pair of electrodes attached on the suprahyoid muscles horizontally just above both ends of the hyoid bone. The other pair of electrodes was attached vertically on the infrahyoid muscles at the midline inferior to the hyoid bone for these patients. In Group C, all electrodes were attached vertically along the midline after verifying the thyroid notch by palpation. The first electrode was placed across the hyoid bone above the thyroid notch and the second electrode was placed immediately superior to the first. The third electrode was placed inferior to the thyroid cartilage and the fourth electrode was placed directly inferior to the third (
Fig. 1
Image shows the placement of neuromuscular electrical stimulation electrodes, with patients being randomised into three groups based on positioning of electrodes on the muscles above and below the hyoid bone, (a) Group A, (b) Group B and (c) Group C.
The electrical stimulation unit (VitalStim®; Chattanooga Group, Hixson, TN, USA) used in this study provided a pulse rate of 80 Hz, with biphasic pulse duration of 300 µs. The amplitude of the electric current (range 0–25 µA) could be adjusted independently for each of the two stimulation channels. The skin in the submental and laryngeal regions was cleaned using a sterile alcohol swab, and two pairs of bipolar surface electrodes were attached. After placing the electrodes on the patient’s neck, stimulation intensity for each pair of electrodes was increased in increments of 0.5 µA until the patient reported feeling a tingling sensation. Then, the stimulation intensity for each channel was increased until muscle contraction was visible.
The patients performed effortful swallowing training simultaneously with NMES. Specifically, patients were repeatedly asked to forcefully swallow their saliva every ten seconds during stimulation in order to elevate the hyolaryngeal complex, followed by a ten-second rest period. Each intervention session lasted 20 minutes and five sessions were performed weekly for four weeks.
The effect of NMES electrode placement was assessed in terms of the Functional Dysphagia Scale (FDS) and Dysphagia Outcome and Severity Scale (DOSS) scores. The FDS is a 100-point scale evaluating the oral (FDS-O) and pharyngeal (FDS-P) phases of swallowing. The components of FDS-O are lip closure, bolus formation, residue in the oral cavity and oral transit time. The components of FDS-P are triggering of pharyngeal swallow, laryngeal elevation and epiglottic closure, nasal penetration, residue in the valleculae, residue in the pyriform sinuses, coating of the pharyngeal wall after swallowing, and pharyngeal transit time.(18) The DOSS is a simple seven-point scale that evaluates the level of independence, tolerated diet consistency and extent of nutritional restrictions.(19)
VFSS was performed to evaluate dysphagia at baseline and after the intervention. While seated, each patient was asked to swallow 6 mL of diluted barium three times. The patients were observed in the lateral and anteroposterior planes. All procedures were recorded in a digital video file and analysed by two physiatrists. The FDS and DOSS scores were established based on the VFSS findings.
All statistical analyses were conducted using IBM SPSS Statistics version 19 for Windows (IBM Corp, Armonk, NY, USA). The Wilcoxon signed-rank test was used for within-group comparisons of the changes in scores following intervention. The Kruskal-Wallis test was used for between-group comparisons of intervention-induced changes. Post-hoc comparisons were made using Bonferroni-corrected Mann-Whitney U tests. Results were considered statistically significant if p < 0.05 on the Wilcoxon signed-rank test or Kruskal-Wallis test. The significance level of the post-hoc comparison was 0.017 (0.05/3).
RESULTS
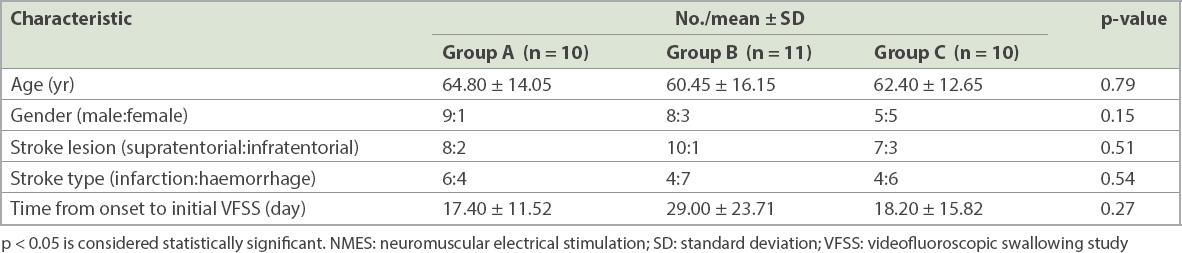
31 patients, who consented to participate, were randomised into three intervention groups: Group A (n = 10); Group B (n = 11); and Group C (n = 10). The general characteristics of patients are summarised in
Table I
Characteristics of patients with dysphagia undergoing NMES treatment.
At baseline, there was no significant difference among the groups regarding FDS (p = 0.865), FDS-O (p = 0.053) or FDS-P (p = 0.589). In all three groups, all FDS scores (overall, FDS-O and FDS-P) improved significantly after the intervention (p < 0.01;
Table II
Within-group comparisons of scores of patients before and after NMES treatment.
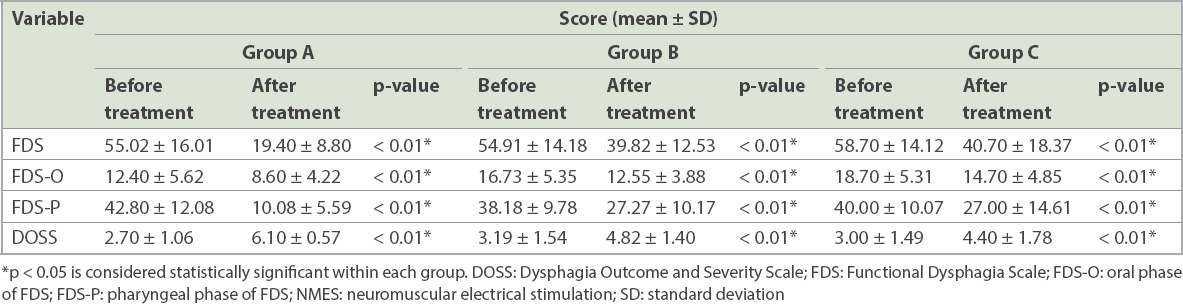
Table III
Between-group comparisons of scores after NMES treatment.
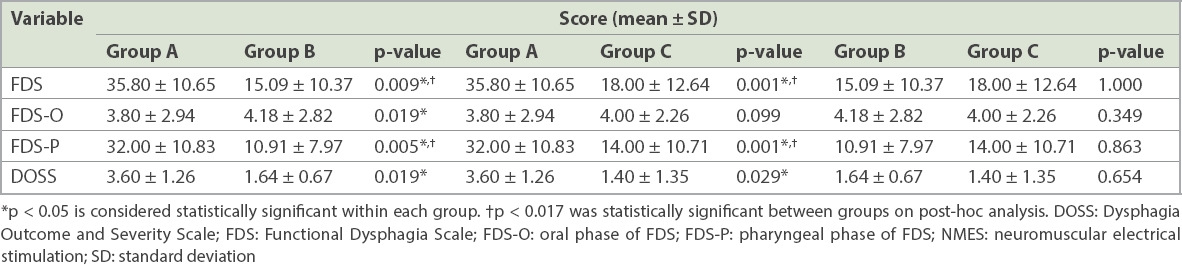
Fig. 2
Charts show the mean differences of (a) FDS scores, (b) FDS-O scores, (c) FDS-P scores and (d) DOSS scores between groups treated under different electrode placements. *p < 0.017 is considered statistically significant between the groups on post-hoc analysis. DOSS: Dysphagia Outcome and Severity Scale; FDS: Functional Dysphagia Scale; FDS-O: oral phase of FDS; FDS-P: pharyngeal phase of FDS
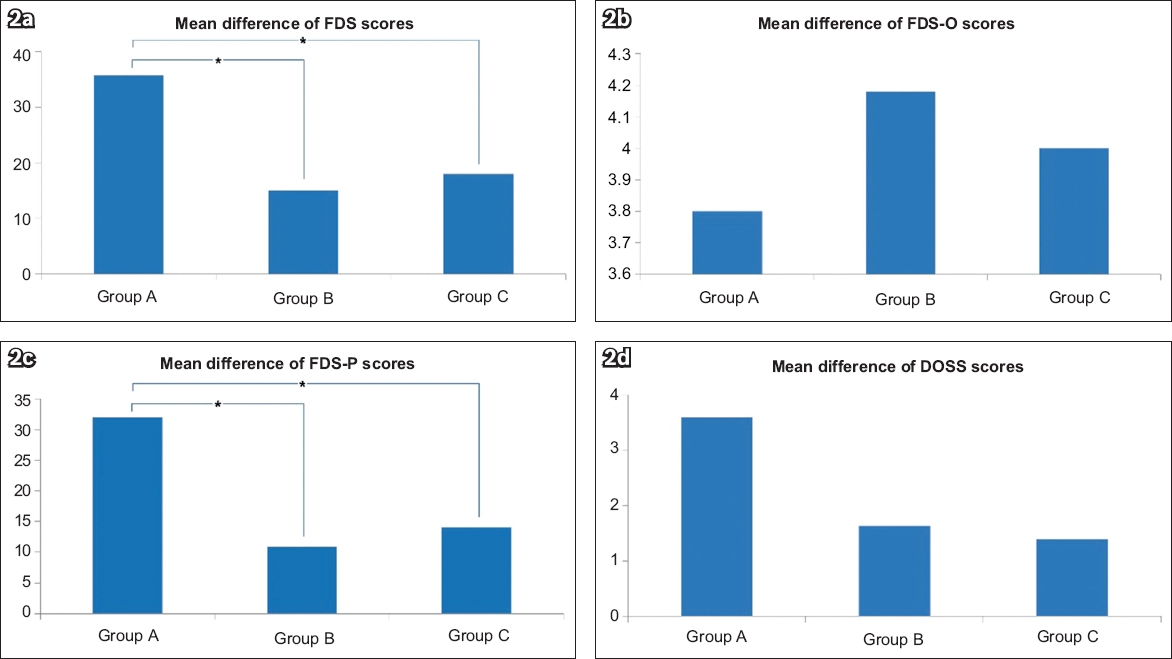
At baseline, no significant difference between the groups was noted in terms of DOSS scores (p = 0.734). All three groups had significant improvement in DOSS scores after the intervention (p < 0.01;
DISCUSSION
We investigated the effect of NMES combined with effortful swallowing training for post-stroke dysphagia in three groups of patients with distinct placements of electrodes. Although FDS and DOSS scores showed significant improvement after the intervention for all three electrode placements, our results indicated that horizontal electrode placement on the suprahyoid and infrahyoid muscles (Group A) was more beneficial.
NMES is commonly used in therapy for swallowing problems. Lim et al suggested that conventional therapy applied together with NMES electrode placement corresponding to Group A was a better treatment for patients with post-stroke dysphagia than conventional therapy alone.(11) Ludlow et al reported that NMES electrode placement corresponding to Group A may oppose hyoid elevation during swallowing.(12) Nam et al and Beom et al found good outcomes for NMES electrode placement corresponding to Group B.(13,14) Carnaby-Mann and Crary reported improvement in clinical swallowing ability and functional oral intake for NMES electrode placement corresponding to Group C.(15) Lim et al reported that NMES electrode placement corresponding to Group C and low-frequency repetitive transcranial magnetic stimulation treatment were effective for treating patients with dysphagia.(16)
In a kinematic analysis of the hyolaryngeal complex during VFSS, Humbert et al reported that the motor effect of stimulation may cause hyolaryngeal lowering.(22) Similarly, Ludlow et al indicated that NMES causes the hyoid bone to move downwards, which may stimulate the patients to make a greater effort to overcome this resistance and swallow.(12) These findings suggest that NMES could be used as a resistance training method, which, when combined with effortful swallowing, may produce a powerful therapeutic effect. Indeed, the kinematic analysis by Park et al demonstrated that effortful swallowing combined with NMES on the sternohyoid muscles lowers the hyoid bone, suggesting that this combined treatment approach may represent a useful rehabilitation strategy.(23)
In our study, a four-week intervention programme with horizontal electrode placement above and below the hyoid bone (in Group A) provided significantly better improvement in FDS and FDS-P scores than other placements (in Groups B and C). Humbert et al investigated ten different surface electrode placements covering the submental and laryngeal regions (including the three placements evaluated in our study) on 29 healthy volunteers (age range 20–60 years) without neurological, phonological, psychiatric, speech or swallowing disorders. The study reported the most significant decrease in both hyoid and laryngeal peak elevation for NMES electrode placement corresponding to Group A.(22) Taken together, these findings suggest that horizontal electrode placement above and below the hyoid bone may indeed be the optimal strategy to decrease hyoid and laryngeal peak elevation, which, when combined with effortful swallowing, would produce the most effective rehabilitation via resistance training.
The present study had several limitations. First, the sample size was small and study period short. Thus, our findings may not be generalisable to long-term rehabilitation programmes and large patient populations. Future studies with larger sample size and longer study period are necessary. Second, we did not have a control group. Our study was designed more to focus on investigating the most effective electrode placement than the effectiveness of NMES itself. Previous meta-analyses have concluded that NMES tends to improve swallowing function,(24,25) and therefore we did not include a control group in our study. Lastly, we evaluated dysphagia by using VFSS without kinematic analysis. Additional studies using kinematic analysis of VFSS would be necessary to identify the mechanism that would explain how NMES improves swallowing function.
Despite its limitations, the present study has shown that horizontal placement of electrodes above and below the hyoid bone is more effective than other placements for the treatment of post-stroke dysphagia using NMES therapy combined with effortful swallowing. This rehabilitation strategy could be an effective approach for the treatment of post-stroke patients with dysphagia.


